Periventricular diffusivity reflects APOE ε4-modulated amyloid accumulation and cognitive impairment in the Alzheimer's disease continuum
- PMID: 40965291
- PMCID: PMC12444947
- DOI: 10.1002/alz.70659
Periventricular diffusivity reflects APOE ε4-modulated amyloid accumulation and cognitive impairment in the Alzheimer's disease continuum
Abstract
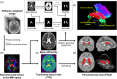
Introduction: Altered glymphatic-related fluid dynamics are increasingly recognized as a feature of Alzheimer's disease (AD). We generalized an established diffusion imaging framework to quantify periventricular diffusivity (PVeD), hypothesizing that fast diffusion signals in the periventricular region can reflect amyloid beta (Aβ) deposition across the AD continuum.
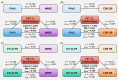
Methods: Participants from two multi-site cohorts (n = 440 and 414), comprising cognitively unimpaired individuals, those with mild cognitive impairment, and patients with AD, were included. We tested and validated the association of PVeD with Aβ burden and core AD characteristics.
Results: Lower PVeD was extensively associated with greater Aβ burden, neurodegeneration, cognitive impairment, and clinical severity in the clinical cohort. Importantly, the relationship between PVeD and Aβ burden was significantly modulated by apolipoprotein E (APOE) ε4 status; APOE ε4 carriers exhibited a replicable stronger negative association. Baseline PVeD also predicted longitudinal cognitive decline.
Discussion: These findings suggest that periventricular diffusion signals reflect APOE ε4-modulated Aβ burden and cognitive decline in AD.
Highlights: An automated method for quantifying periventricular diffusivity (PVeD) is developed. Lower PVeD is associated with higher amyloid load only in a mild cognitive impairment-dominant cohort. Higher amyloid burden may mediate the link between lower PVeD and poorer cognitive outcomes in the clinical cohort. Apolipoprotein E ε4 carriers show a reproducibly stronger inverse PVeD-amyloid association than non-carriers. Baseline PVeD can predict longitudinal Mini-Mental State Examination decline in two independent cohorts.
Keywords: Alzheimer's disease; amyloid imaging; apolipoprotein E ε4; cognitive decline; dementia; diffusion tensor image analysis along the perivascular space; diffusion tensor imaging; diffusivity; perivascular space; periventricular area.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare that they have no financial/non‐financial and direct/potential conflicts of interest. Author disclosures are available in the supporting information.
Figures





References
-
- Livingston G, Huntley J, Liu KY, et al. Dementia prevention, intervention, and care: 2024 report of the Lancet standing commission. The Lancet. 2024;404(10452):572‐628. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R012RF1AG025516/AG/NIA NIH HHS/United States
- R01AG067018/AG/NIA NIH HHS/United States
- P01AG025204/AG/NIA NIH HHS/United States
- R01AG085566/AG/NIA NIH HHS/United States
- R01AG063752/AG/NIA NIH HHS/United States
- RS-2019-NR040055/Ministry of Science and ICT, Republic of Korea
- 2024-ER0505-00/Korea National Institute of Health
- RS-2024-00339665/Ministry of Health & Welfare and Ministry of Science and ICT, Republic of Korea
- HR21C1003/Ministry of Health & Welfare, Republic of Korea
- HR22C1734/Ministry of Health & Welfare, Republic of Korea
- RS-2024-00406876/Ministry of Health & Welfare, Republic of Korea
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

