Overview of European Practices for Management of Tyrosinemia Type 1: Towards European Guidelines
- PMID: 40965374
- PMCID: PMC12445258
- DOI: 10.1002/jimd.70089
Overview of European Practices for Management of Tyrosinemia Type 1: Towards European Guidelines
Abstract
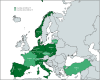
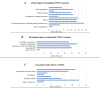
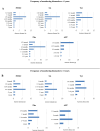
The introduction of nitisinone (NTBC) and newborn screening for Tyrosinemia type 1 (TT1) enabled preemptive treatment of patients, thereby significantly improving outcomes by preventing liver, kidney, and neurological issues. Treatment goals have shifted from emergency treatment to long-term care. To evaluate the risk of developing complications with aging, due to TT1 itself or its treatment, long-term follow-up is essential. In 2014, an overview of TT1 management practices in Europe was published. Within the Metabolic European Reference Network's subnetwork on amino-and-organic acidurias (MetabERN-AOA), we considered it important to give an update on current TT1 management practices in Europe. An online survey study was performed among members of the MetabERN-AOA subnetwork, and participants of a workshop on TT1 at the European Metabolic Group Meeting of Nutricia. Findings were compared to existing data from the aforementioned publication from 2014 and previously published recommendations. Thirty-two centers (16 European countries) completed the survey. Both consistencies and inconsistencies in TT1 management were seen. Inconsistencies were observed in the frequency and methods of follow-up, dosing of NTBC, and target ranges of biochemical markers. Compared to 2014, key differences included an increased number of patients detected by newborn screening, lower NTBC dosing, and a shift from interest in mainly hepatic to hepatic and neurocognitive outcomes. These results align with trends seen in TT1 recommendations over the years. In addition to numerous consistencies, many aspects in TT1 management still differ widely across Europe, suggesting the need for uniform guidance in clinical management beyond existing recommendations.
Keywords: NTBC; guidelines; management; tyrosinemia type 1.
© 2025 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
All co‐authors, excluding A.M.K., are members of the MetabERN and AOA subnetwork. F.J.V.S. has received research grants, advisory board fees, and/or speaker's honoraria from Agios, Alexion, AlltRNA, Applied Pharma Research, Arla Food Int., Biomarin, Beatrix Research Fund, ESPKU, Nestle‐Codexis Alliance, Moderna, Nutricia, NPKUA, NPKUV, Pluvia Biotech, PTC Therapeutics, Rivium Medical BV, Sobi, Tyrosinemia Foundation, Vitaflo, and ZONMW. The other authors declare no conflicts of interest.
Figures



References
-
- Van Spronsen F. J., Thomasse Y., Smit G. P. A., et al., “Hereditary Tyrosinemia Type I: A New Clinical Classification With Difference in Prognosis on Dietary Treatment,” Hepatology 20, no. 5 (1994): 1187–1191. - PubMed
-
- Lindstedt S., Holme E., Lock E. A., Hjalmarson O., and Strandvik B., “Treatment of Hereditary Tyrosinaemia Type I by Inhibition of 4‐Hydroxyphenylpyruvate Dioxygenase,” Lancet 340, no. 8823 (1992): 813–817. - PubMed
-
- Holme E. and Lindstedt S., “Tyrosinaemia Type I and NTBC (2‐(2‐Nitro‐4‐Trifluoromethylbenzoyl)‐1,3‐Cyclohexanedione),” Journal of Inherited Metabolic Disease 21 (1998): 507–517. - PubMed
-
- Larochelle J., Alvarez F., Bussières J. F., et al., “Effect of Nitisinone (NTBC) Treatment on the Clinical Course of Hepatorenal Tyrosinemia in Québec,” Molecular Genetics and Metabolism 107, no. 1–2 (2012): 49–54. - PubMed
-
- Therrell B. L., Padilla C. D., Loeber J. G., et al., “Current Status of Newborn Screening Worldwide: 2015,” Seminars in Perinatology 39, no. 3 (2015): 171–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

