Outcomes of Critically Ill Adult Patients With Acute Encephalitis
- PMID: 40965884
- PMCID: PMC12447255
- DOI: 10.1001/jamanetworkopen.2025.32478
Outcomes of Critically Ill Adult Patients With Acute Encephalitis
Abstract
Importance: Functional outcomes and long-term recovery after severe encephalitis are not well characterized.
Objective: To determine the incidence of functional disability or death at 3 months and to describe recovery trajectories through 1 year after encephalitis.
Design, setting, and participants: This prospective multicenter cohort study was conducted across 31 French centers from October 2017 to April 2021 and included adults with probable or confirmed encephalitis and clear cerebrospinal fluid findings requiring care in the intensive care unit. Data analysis was performed between May 2023 and June 2025.
Exposure: Causes of encephalitis were categorized into 4 different groups: infectious, autoimmune, other causes, and unknown origin.
Main outcomes and measures: The primary end point was an unfavorable outcome at 3 months, defined by a modified Rankin scale score of 3 to 6, indicating moderate to severe disability or death.
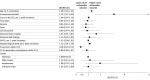
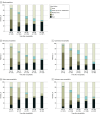
Results: Among the 310 patients included (median [IQR] age, 60 [43-70] years; 177 male [57.1%]), 123 (39.7%) were diagnosed with infectious encephalitis, 42 (13.5%) with autoimmune encephalitis, 37 (11.9%) with other encephalitis causes, and 108 (34.8%) with encephalitis of unknown origin. Overall, 161 patients (51.9%; 95% CI, 46.2%-57.6%) had an unfavorable outcome at 3 months, including 84 deaths (27.1%). Independent factors associated with unfavorable outcome included age (odds ratio [OR] per 5-year increment, 1.28, 95% CI, 1.16 to 1.41) and immunocompromised status (OR, 3.12; 95% CI, 1.57 to 6.40), while intravenous acyclovir on the day of ICU admission was associated with a favorable outcome (OR, 0.38; 95% CI, 0.20 to 0.72). The proportion of patients achieving functional independence remained stable from 3 months to 1 year (difference in proportions, 1.1%; 95% CI, -6.9% to 9.2%). Analyses based on encephalitis cause groups revealed that patients with autoimmune encephalitis showed significant improvement through 1 year (difference in proportions, 8.9%; 95% CI, 1.2% to 16.6%), whereas no significant changes were seen in patients with infectious causes (difference in proportions, 1.2%; 95% CI, -6.9% to 9.2%), other causes (difference in proportions, 1.2%; 95% CI: -6.8% to 9.2%), or unknown origin (difference in proportions, -1.9%; 95% CI: -10.0% to 6.2%).
Conclusions and relevance: In this cohort study of adults with severe encephalitis requiring intensive care, one-half of patients had an unfavorable outcome at 3 months. Functional recovery at 1 year varied by cause of encephalitis, with patients with autoimmune encephalitis experiencing more favorable outcomes than those with other causes, suggesting a possible role for targeted long-term support in certain cases.
Conflict of interest statement
Figures

References
-
- Venkatesan A, Tunkel AR, Bloch KC, et al. ; International Encephalitis Consortium . Case definitions, diagnostic algorithms, and priorities in encephalitis: consensus statement of the international encephalitis consortium. Clin Infect Dis. 2013;57(8):1114-1128. doi: 10.1093/cid/cit458 - DOI - PMC - PubMed
-
- Granerod J, Ambrose HE, Davies NW, et al. ; UK Health Protection Agency (HPA) Aetiology of Encephalitis Study Group . Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835-844. doi: 10.1016/S1473-3099(10)70222-X - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

