Early Blood Pressure Targets in Acute Spinal Cord Injury: A Randomized Clinical Trial
- PMID: 40965887
- PMCID: PMC12447234
- DOI: 10.1001/jamanetworkopen.2025.25364
Early Blood Pressure Targets in Acute Spinal Cord Injury: A Randomized Clinical Trial
Abstract
Importance: Early blood pressure management is central to neurologic resuscitation of spinal cord injury; however, the role of augmented blood pressure is unclear.
Objective: To compare the efficacy and safety of augmented vs conventional blood pressure on 6-month neurologic outcomes after acute spinal cord injury.
Design, setting, and participants: This multicenter randomized clinical trial took place from October 3, 2017, to July 26, 2023, and assessed patients 18 years or older with spinal cord injury followed up for 6 months at 13 large US trauma centers.
Interventions: Patients were equally randomized to augmented (>85-90 mm Hg) or conventional (>65-70 mm Hg) mean arterial pressure for 7 days or until intensive care unit discharge.
Main outcomes and measures: Primary end points were change in motor and sensory American Spinal Injury Association Impairment Scale scores from baseline to 6 months. Safety end points included organ dysfunction and complications.
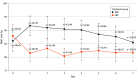
Results: The trial randomized 92 patients (mean [SD] age, 53.78 [18.74] years; 76 [83%] male). At 6 months, 38 patients had completed follow-up and 15 had died. Among survivors, there were no mean (SD) differences in change from baseline in upper extremity motor scores (34.95 [3.25] vs 32.95 [3.65]; difference, 2.48; 95% CI, -5.93 to 10.90; P = .55), lower extremity motor scores (18.53 [4.62] vs 19.95 [4.59]; difference, -4.56; 95% CI, -16.11 to 7.03; P = .43), or total sensory scores (108.47 [12.49] vs 130.89 [14.87]; difference, -32.00; 95% CI, -65.40 to 1.40; P = .06) comparing the augmented and conventional groups. The augmented group had higher mean (SD) modified Sequential Organ Failure Assessment scores (excluding cardiovascular components) at day 3 (1.65 [1.79] vs 0.80 [1.10]; difference, 0.85; 95% CI, 0.23-1.47; P = .008) and day 6 (1.55 [1.82] vs 0.80 [1.35]; difference, 0.74; 95% CI, 0.05-1.44; P = .04), longer mechanical ventilatory support (9.44 [15.27] vs 3.78 [8.42] days; difference, 5.67 days; 95% CI, 0.48-10.85 days; P = .03), and more respiratory complications (36 [78%] vs 18 [39%]; risk difference, 40%; 95% CI, 22%-58%; P < .001) than the conventional group. No differences in mortality or other secondary outcomes were observed.
Conclusions: Although underpowered, this randomized clinical trial of patients with spinal cord injury did not demonstrate better neurologic recovery comparing early augmented and conventional blood pressure and calls this practice into question. Further study is needed to identify groups who may benefit from augmenting blood pressure and determine potential harm mechanisms.
Trial registration: ClinicalTrials.gov Identifier: NCT02878850.
Conflict of interest statement
Figures

Comment in
References
-
- World Health Organization. Spinal Cord Injury. Accessed August 4, 2024. https://www.who.int/news-room/fact-sheets/detail/spinal-cord-injury
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

