Covered versus uncovered endoluminal stenting in the acute management of obstructing colorectal cancer in the palliative setting: randomized clinical trial (CReST2)
- PMID: 40966674
- PMCID: PMC12448851
- DOI: 10.1093/bjs/znaf117
Covered versus uncovered endoluminal stenting in the acute management of obstructing colorectal cancer in the palliative setting: randomized clinical trial (CReST2)
Abstract
Background: Around 15% of people with colon cancer present with an obstruction. Stenting is appropriate for patients unfit for surgery and/or those with advanced cancer. Patients are living longer with advanced colon cancer; stent design (covered versus uncovered) may influence stent re-intervention and quality of life (QoL).
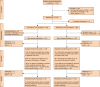
Methods: CReST2 is a phase III multicentre RCT. Patients were randomized 1 : 1 to receive either a covered or uncovered stent. Patients and all medical personnel except the person placing the stent were blinded to allocation. Treatment allocation was via a central randomization service, minimized for: age (≤70 years, >70 years), WHO performance status, tumour site, and indication for palliation. Co-primary endpoints were stent patency up to 6 months after randomization and QoL at 3 months (30 days for patients who died before 3 months) from randomization measured using the European Organisation for Research and Treatment of Cancer (EORTC) QLQ-C30 global health score. Secondary endpoints were stenting success rate, rates of short-term (30 days), intermediate-term (1-3 months), and long-term (3-6 months) stent-related complications, stent-related complication rates of patients undergoing chemotherapy within 6 months after randomization, cumulative frequency of stoma formation, survival at 6 months, and overall survival.
Results: A total of 377 patients were randomised across 28 sites, in whom stenting was unsuccessful in 47 (12.5%) patients (27 of 188: 14.4% covered and 20 of 186: 10.7% uncovered stents). Stent patency at 6 months in stented patients was 117 of 161 (72.7%, covered) and 136 of 166 (81.9%, uncovered) (adjusted HR 1.48, 97.5% confidence interval (c.i.): 0.86-2.54). In this stented population, 216 patients (66.1%) contributed to QoL assessment at 3 months with mean(s.d.) QLQ-C30 global health scores of 54.1(23.9) and 51.6(25.4) in the covered and uncovered groups respectively (adjusted mean difference 1.63, 97.5% c.i. -5.85-9.11). The total numbers of patients experiencing at least one complication in the first 6 months after randomization were 42 of 161 (26.1%) for covered stents and 29 of 166 (17.5%) for uncovered stents. Stent migration was the most common complication and was higher in the covered group. In the covered group and the uncovered group, 44 of 161 (27.3%) and 40 of 166 (24.1%) patients respectively received chemotherapy up to 6 months after randomization. There was a low risk of late perforation associated with both types of stent.
Conclusion: There appears to be greater prolonged stent patency and less stent failure with uncovered stents. QoL is unaffected by stent design.
Registration number: ISRCTN54834267.
© The Author(s) 2025. Published by Oxford University Press on behalf of BJS Foundation Ltd.
Conflict of interest statement
Members of the CReST2 Collaborative Group are co-authors of this study and are listed under the heading Collaborators.
Figures

References
-
- National Bowel Cancer Audit Report. 2015. https://www.nboca.org.uk/reports/annual-report-2015/ (accessed 18 July 2025)
-
- Flor-Lorente B, Báguena G, Frasson M, García-Granero A, Cervantes A, Sanchiz V et al. Self-expanding metallic stent as a bridge to surgery in the treatment of left colon cancer obstruction: cost-benefit analysis and oncologic results. Cir Esp 2017;95:143–151 - PubMed
-
- Saito S, Yoshida S, Isayama H, Matsuzawa T, Kuwai T, Maetani I et al. A prospective multicenter study on self-expandable metallic stents as a bridge to surgery for malignant colorectal obstruction in Japan: efficacy and safety in 312 patients. Surg Endosc 2016;30:3976–3986 - PubMed
-
- Li C-Y, Guo S-B, Wang N-F. Decompression of acute left-sided malignant colorectal obstruction: comparing transanal drainage tube with metallic stent. J Clin Gastroenterol 2014;48:e37–e42 - PubMed
-
- Foo CC, Poon SHT, Chiu RHY, Lam WY, Cheung LC, Law WL. Is bridge to surgery stenting a safe alternative to emergency surgery in malignant colonic obstruction: a meta-analysis of randomized control trials. Surg Endosc 2019;33:293–302 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

