A Systematic Review of Algorithms for Identifying Pediatric Neurodevelopmental Outcomes
- PMID: 40967198
- PMCID: PMC12445936
- DOI: 10.1002/pds.70196
A Systematic Review of Algorithms for Identifying Pediatric Neurodevelopmental Outcomes
Abstract
Purpose: Investigating pediatric neurodevelopmental outcomes (NDO) in studies using secondary data is often challenging due to heterogeneous clinical definitions and medical coding systems. This study aims to identify the algorithms used to define NDO in studies using electronic healthcare data through a systematic literature review.
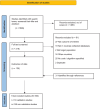
Methods: A search strategy was developed to identify studies on NDO that describe phenotype algorithms from January 1, 2010, to March 10, 2025. The search strategy included terms to identify studies containing algorithms for NDO as an outcome, routinely collected healthcare data, epidemiologic designs likely to incorporate algorithms, and pregnant individuals and/or infants/children. Two independent reviewers assessed eligibility criteria and performed data extraction, with inconsistencies reviewed by a third reviewer. Descriptive statistics were used to summarize categorical and continuous variables appropriately.
Results: The review included 156 publications that implemented algorithms for NDO, with 18 of these studies validating the outcomes. Most publications studied autism spectrum disorder (ASD) (n = 103, 65.6%) and attention deficit hyperactivity disorder (ADHD) (n = 72, 45.9%) either as a single outcome or as a composite.
Conclusions: Instead of presenting NDO as a composite outcome, it is recommended to present multiple single outcomes. Validated outcomes in data from Nordic countries demonstrate a high positive predictive value when using one code for diagnoses, while more complex algorithms are required for US data. Clearly detailing and establishing the time of assessment for each NDO is critical to inform valid epidemiological estimates.
Keywords: attention‐deficit hyperactivity disorder; autism; neurodevelopmental; pharmacoepidemiology; pregnancy; real‐world evidence; validation.
© 2025 The Author(s). Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
An earlier version of this article was presented at the 2024 International Society and Pharmacoepidemiology Annual Meeting, ISPE 2024, Berlin.
The authors declare no conflicts of interest.
References
-
- “Diagnostic and Statistical Manual of Mental Disorders. Psychiatry Online. DSM Library,” accessed November 4, 2024, 10.1176/appi.books.9780890425787. - DOI
-
- Schilow W. F. and Nossack A., “The Use of Ultrasound‐Treated Amd Complete Bordetella bronchiseptica Antigens in an Indirect Enzyme Immunoassay,” Archiv für Experimentelle Veterinärmedizin 42, no. 3 (1988): 324–327. - PubMed
-
- Hjorth S., Bromley R., Ystrom E., Lupattelli A., Spigset O., and Nordeng H., “Use and Validity of Child Neurodevelopment Outcome Measures in Studies on Prenatal Exposure to Psychotropic and Analgesic Medications—A Systematic Review,” PLoS One 14, no. 7 (2019): e0219778, 10.1371/journal.pone.0219778. - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical