High flow oxygen for vaso-occlusive crisis: a multicentre, prospective, randomised, multi-arm, multi-stage clinical trial (OSONE)
- PMID: 40967654
- PMCID: PMC12458843
- DOI: 10.1136/bmjopen-2025-104564
High flow oxygen for vaso-occlusive crisis: a multicentre, prospective, randomised, multi-arm, multi-stage clinical trial (OSONE)
Abstract
Introduction: Sickle cell disease (SCD) is due to the mutation of haemoglobin (Hb), from HbA to HbS and characterised by recurrent vaso-occlusive crises (VOC), which can progress to acute chest syndrome (ACS), a leading cause of death in adults with SCD. Hypoxia is a key modifiable factor in the polymerisation of HbS and the pathogenesis of VOC. High-flow nasal oxygen (HFNO) delivers humidified gas at high oxygen concentrations and flow rates: the former may reverse sickling (metabolic effect) to accelerate VOC resolution and prevent ACS, while the latter may reduce the risk of ACS by mitigating hypercapnia and generating positive airway pressure that limits hypoventilation and atelectasis (pulmonary effect). The study hypothesises that HFNO is a safe and effective strategy for treating VOC and preventing secondary ACS, and will assess this using a multi-arm multi-stage (MAMS) trial design.
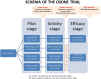
Methods and analysis: This is a prospective, multicentre, randomised, open-label controlled trial following an MAMS design with three phases and four arms: one control (low-flow oxygen) and three HFNO intervention arms with varying fraction of inspired oxygen levels (low, intermediate, high). The pilot stage will assess safety and feasibility, using the rate of cardiac and neurological events as the primary endpoint. In the activity stage, arms demonstrating acceptable safety will be compared for efficacy based on the rate of VOC resolution without complications by day 5, allowing selection of the most promising arm. The final efficacy stage will compare the selected HFNO strategy to control, with prevention of secondary ACS by day 14 as the primary endpoint. The study aims to enrol up to 350 VOC episodes in total.
Ethics and dissemination: The study has been granted ethical approval (CPP SUD MEDITERRANEE IV). Following the provision of informed consent, patients will be included in the study. The results will be submitted for publication in peer-reviewed journals.
Trial registration number: NCT03976180.
Keywords: Anaemia; Hyperoxia; Randomized Controlled Trial.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: AMD reports fees (lectures and research) from Fisher & Payckel. EA reports personal fees from GBT, personal fees from Hemanext, both unrelated to the present study. GM reports fees for lectures and research grants from AZ, Gilead, Pfizer, Viiv, Astellas, all unrelated to the present study. CK reports fees from CSL BEHRING, unrelated to the present study. PC reports fees from GBT, Theravia, Agios unrelated to the present study. J-BA declares the receipt of research funding from Addmedica/Theravia, GBT-Pfizer, Novartis, and Vertex and Agios. SG reports support from Pharma Dom for attending a meeting, unrelated to the submitted work. PB receives consulting fees and honoraria for lectures from Agios. He is the co-founder of the INNOVHEM startup. Other authors report no competing interest.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous