Stabilizing a mammalian RNA thermometer confers neuroprotection in subarachnoid hemorrhage
- PMID: 40968270
- PMCID: PMC12446472
- DOI: 10.1038/s41467-025-63911-3
Stabilizing a mammalian RNA thermometer confers neuroprotection in subarachnoid hemorrhage
Abstract
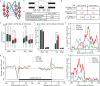
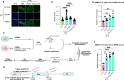
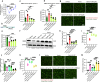
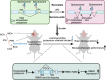
Mammals tightly regulate their core body temperature, yet how cells sense and respond to small temperature changes remains incompletely understood. Here, we discover RNA G-quadruplexes (rG4s) as key thermosensors enriched near splice sites of cold-repressed exons. These thermosensing RNA structures, when stabilized, mask splice sites, reducing exon inclusion. Specifically, rG4s near splice sites of a cold-repressed poison exon in the neuroprotective RBM3 are stabilized at low temperatures, leading to exon exclusion. This enables evasion of nonsense-mediated decay, increasing RBM3 expression at cold. Importantly, stabilizing rG4 through increasing intracellular potassium with an FDA-approved potassium channel blocker, mimics the hypothermic effect on alternative splicing, thereby increasing RBM3 expression, leading to RBM3-dependent neuroprotection in a mouse model of subarachnoid hemorrhage. Our findings unveil a mechanism how mammalian RNAs directly sense temperature and potassium perturbations, integrating them into gene expression programs. This opens new avenues for treating diseases arising from splicing defects and disorders benefiting from therapeutic hypothermia, especially hemorrhagic stroke.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: A patent application (China patent No. 2024105567784) has been filed in relation to this research. There are no other competing interests.
Figures




References
-
- Brown, D. J., Brugger, H., Boyd, J. & Paal, P. Accidental hypothermia. N. Engl. J. Med.367, 1930–1938 (2012). - PubMed
-
- Yuan, F. et al. Z-DNA binding protein 1 promotes heatstroke-induced cell death. Science376, 609–615 (2022). - PubMed
-
- Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med.346, 549–556 (2002). - PubMed
-
- Kollmar, R. et al. Hypothermia reduces perihemorrhagic edema after intracerebral hemorrhage. Stroke41, 1684–1689 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

