Impact of platinum-based chemotherapy and CTLA-4 inhibition on acquired resistance to first-line anti-PD-1/PD-L1 agents in non-small cell lung cancer: a systematic review and reconstructed individual patient data analysis
- PMID: 40969680
- PMCID: PMC12441679
- DOI: 10.1016/j.eclinm.2025.103482
Impact of platinum-based chemotherapy and CTLA-4 inhibition on acquired resistance to first-line anti-PD-1/PD-L1 agents in non-small cell lung cancer: a systematic review and reconstructed individual patient data analysis
Abstract
Background: Acquired resistance is defined as disease progression following an initial response to immune checkpoint inhibitors (ICI). The impact of adding platinum-based chemotherapy (PCT) or anti-CTLA-4 agents to PD-(L)-1 inhibitors on acquired resistance is currently unknown.
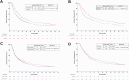
Methods: Systematic research by January 31, 2025 identified randomized clinical trials (RCTs) evaluating first-line ICI as monotherapy (mono-ICI) or in combination with PCT (mono-ICI + PCT), CTLA-4 inhibitors (combo-ICI), or both (combo-ICI + PCT) in metastatic non-small-cell lung cancer (NSCLC). RCTs reporting duration of response (DoR) data were eligible. Acquired resistance rates at 6 and 12 months were estimated from DoR curves. Aggregate data based on type of regimens were reported with risk ratio (RR) and pooled by random effect model. Primary and secondary endpoints were respectively the indirect comparison of acquired resistance risk between PCT-containing versus PCT-free regimens and between anti-CTLA-4 containing versus anti-CTLA-4 free regimens, respectively. Time-to-event outcomes were retrieved from Kaplan-Meier (KM) curves using individual patient data (IPD) and compared by log rank tests. This study was registered to the PROSPERO online platform (CRD42025639320).
Findings: Nineteen RCTs were included. 6- and 12-months acquired resistance rates were 16.5%-34.1% (mono-ICI), 26.4%-47.8% (mono-ICI + PCT), 19.0%-33.0% (combo-ICI), and 28.4%-47.1% (combo-ICI + PCT). A higher risk of acquired resistance was suggested from indirect comparisons of mono-ICI + PCT versus mono-ICI (12-months RR: 1.46, 95% CI 1.23-1.75) and of combo-ICI + PCT versus combo-ICI (12 months RR: 1.36, 95% CI 1.06-1.73). Using the reconstructed patient-level data, median DoR was 5-7 months significantly shorter with PCT-containing compared to PCT-free regimens. The addition of anti-CTLA-4 agents did not impact on acquired resistance.
Interpretation: Although insufficient RCTs reported acquired resistance rates stratified by specific factors influencing DoR (i.e., PD-L1, TMB) for such analyses to be included in this report, the increased acquired resistance risk observed with PCT-containing regimens highlights the potential value of tailoring first-line treatment strategies in NSCLC on the basis of individual risk of resistance to ICI.
Funding: This research did not receive any specific grant from funding agencies.
Keywords: Acquired resistance; CTLA-4 inhibitors; Immunotherapy; Non-small cell lung cancer; Platinum-based chemotherapy.
© 2025 The Author(s).
Conflict of interest statement
Sara Oresti reports Speaker’s fee from AstraZeneca and Roche; Support for attending meetings and/or travel from Jannses, Johnson & Johnson and Takeda. Francesca Rita Ogliari reports Speaker’s fee from Roche, Sanofi, Amgen; Support for attending meetings and/or travel from Amgen, Johnson & Johnson. Raffaele Califano reports grants or contracts from Merck Sharp & Dohme, Roche, PharmaMar, GSK, Janssen, AstraZeneca, Taiho, ArriVent and OSE Immunotherapeutics; consulting fees from Pfizer, Merck Sharp & Dohme, PharmaMar, ArriVent, Roche, Bristol Myers Squibb, AstraZeneca, Biontech, Janssen, GSK and Takeda; payment or honoraria from Beigene, Regeneron, AstraZeneca, Janssen, GSK, Takeda; support for attending meetings and/or travel from Janssen, Takeda; participation on a data safety monitoring board or advisory board for PharmaMar, Janssen, Astrazeneca, Arrivent; leadership or fiduciary role for ESMO Educational Publication Working Group; stock or stock options with Supportive Care UK and LOC at the Christie Private Care. Bulotta Alessandra reports honoraria for a role as consultant and advisory board for Roche, BMS, AstraZeneca and speaker fees for BMS, MSD, AstraZeneca, Ely Lilly. Biagio Ricciuti reports speaker fees from Targeted Oncology, consultancy or participation to advisory board from Regeneron, AstraZeneca, Amgen, Guidepoint Inc. Alessio Cortellini reports grants for consultancies/advisory boards from MSD, BMS, IQVIA, AstraZeneca, REGENERON, Amgen, Daiichi-Sankyo, Access Infinity, Ardelis Health, Alpha Sight, Guidepoint, Roche and Alira Health; speaker fees from AstraZeneca, Pierre-Fabre, MSD, Sanofi/REGENERON; payment for writing/editorial activity from BMS, MSD, Roche; travel support from Sanofi/REGENERON, MSD. Martin Reck reports speaker fees from Amgen, AstraZeneca, Beigene, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, GSK, Mirati, Merck, MSD, Lily, Novartis, Pfizer, Sanofi, Roche, and Regeneron; consultancy or participation to advisory board from Daiichi Sankyo and Sanofi. Alberto Servetto reports honoraria from Eli Lilly, MSD, and Janssen and travel support from Bristol-Myers Squibb and AstraZeneca. Massimo Di Maio reports honoraria from Pfizer, Takeda, AstraZeneca, Janssen, Eisai, Novartis, Roche, Astellas Pharma, MSD Oncology, Boehringer Ingelheim, Viatris, Ipsen; consultancy or participation to advisory boards and direct research funding from AstraZeneca, Pfizer, Takeda, Janssen, Eisai, Novartis, Roche, MSD, Viatris, Ipsen; Research Funding from Tesaro/GlaxoSmithKline. Giuseppe Viscardi reports travel support from AstraZeneca, MSD, Novartis, Sanofi; honoraria for advisory board from Amgen, MSD, Novartis. Ferrara Roberto reports honoraria for advisory board from MSD, Beigene, AstraZeneca, BMS, Sanofi, Johnson & Johnson.
Figures



References
-
- Proto C., Ferrara R., Signorelli D., et al. Choosing wisely first line immunotherapy in non-small cell lung cancer (NSCLC): what to add and what to leave out. Cancer Treat Rev. 2019;75:39–51. - PubMed
-
- Di Federico A., Alden S.L., Smithy J.W., et al. Intrapatient variation in PD-L1 expression and tumor mutational burden and the impact on outcomes to immune checkpoint inhibitor therapy in patients with non-small-cell lung cancer. Ann Oncol. 2024;35:902–913. - PubMed
-
- Wang X., Lamberti G., Di Federico A., et al. Tumor mutational burden for the prediction of PD-(L)1 blockade efficacy in cancer: challenges and opportunities. Ann Oncol. 2024;35:508–522. - PubMed
-
- Viscardi G., Tralongo A.C., Massari F., et al. Comparative assessment of early mortality risk upon immune checkpoint inhibitors alone or in combination with other agents across solid malignancies: a systematic review and meta-analysis. Eur J Cancer. 2022;177:175–185. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

