Magnetic catalyst marvels: a sustainable approach to highly substituted imidazole synthesis
- PMID: 40970084
- PMCID: PMC12443059
- DOI: 10.1039/d5na00368g
Magnetic catalyst marvels: a sustainable approach to highly substituted imidazole synthesis
Abstract
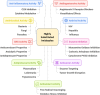
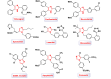
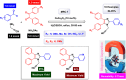
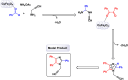
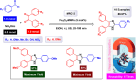
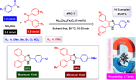
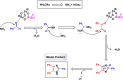
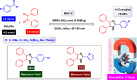
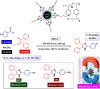
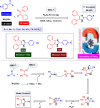
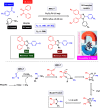
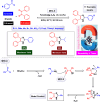
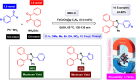
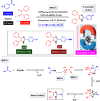
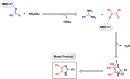
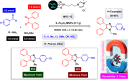
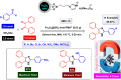
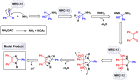
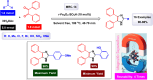
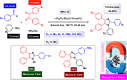
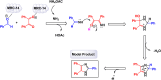
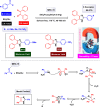
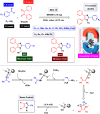
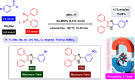
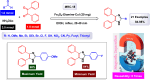
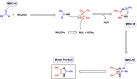
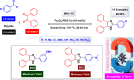
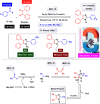
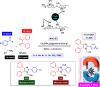
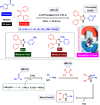
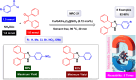
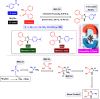
This comprehensive review delves into the recent advancements in magnetic catalyst technology, mainly focusing on their application in facilitating greener and more efficient synthetic routes for imidazole derivatives. This manuscript assesses various magnetic catalyst systems, examining their synthesis, functionalization, and mechanistic roles in promoting imidazole formation. Special attention is given to the environmental benefits of using magnetic catalysts, such as reduced solvent use, lower energy consumption, and enhanced recyclability, which align with sustainable chemistry principles. The unique properties of magnetic catalysts, including their easy recovery via external magnetic fields and reusability without significant loss of activity, are highlighted as key factors driving the synthetic processes' sustainability and economic viability. Furthermore, the review discusses the challenges and limitations currently faced in this realm and proposes future directions for research, including the development of novel magnetic catalyst compositions and the exploration of their utility in other heterocyclic syntheses. By providing a detailed analysis of existing data and suggesting pathways for innovation, this review aims to inspire continued advancement in sustainable catalysis, promising to revolutionize the synthesis of highly substituted imidazoles and expand their potential applications in various industries. This manuscript is a crucial resource for researchers in catalysis and sustainable chemistry. It underscores the broader implications of magnetic catalysts in enhancing green manufacturing practices in the chemical industry, thereby contributing to global sustainability goals.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








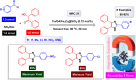
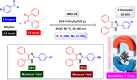

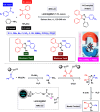
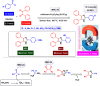


References
-
- Javahershenas R. Recent advances in the application of deep eutectic solvents for the synthesis of Spiro heterocyclic scaffolds via multicomponent reactions. J. Mol. Liq. 2023;385:122398. doi: 10.1016/j.molliq.2023.122398. - DOI
-
- Mahato N. Agarwal P. Mohapatra D. Sinha M. Dhyani A. Pathak B. Tripathi M. K. Angaiah S. Biotransformation of Citrus Waste-II: Bio-Sorbent Materials for Removal of Dyes, Heavy Metals and Toxic Chemicals from Polluted Water. Processes. 2021;9:1544. doi: 10.3390/pr9091544. - DOI
Publication types
LinkOut - more resources
Full Text Sources

