Beyond Risk Factors: Rethinking Hepatitis B and Hepatitis C Screening in Primary Care
- PMID: 40970664
- PMCID: PMC12447784
- DOI: 10.1111/liv.70330
Beyond Risk Factors: Rethinking Hepatitis B and Hepatitis C Screening in Primary Care
Abstract
Introduction: Most international guidelines recommend screening for hepatitis B virus (HBV) and hepatitis C virus (HCV) only for individuals with risk factors or elevated ALT levels. However, this approach may not suffice to eradicate viral hepatitis. This study evaluates the application of EASL HBV and HCV screening guidelines in primary care centres (PCCs).
Methods: The study included two components: (1) A retrospective review (January 2021-March 2023) of microbiology data to determine testing rates, analyse clinical characteristics, and assess management; and (2) A prospective intervention (March-April 2024) involving HBV and HCV screening and risk factor surveys for all adults attending two PCCs for blood collection.
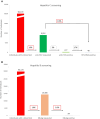
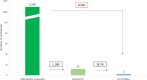
Results: In the retrospective analysis of 90 170 patients, HBV and HCV screening rates were 16% and 10%, respectively. Among HBsAg-positive patients (n = 84 (0.5%)), 67% lacked risk factors or elevated ALT. Among anti-HCV-positive (n = 277 (3%)) and HCV RNA-positive (n = 45 (0.5%)) patients, 54% and 46% respectively lacked these indicators. In the prospective study of 1030 patients (mean age 55, 39.6% men, 73% Spanish), anti-HCV was detected in 1.16% of cases (HCV RNA in 0.19%). Of these, 67% lacked risk factors or elevated ALT. No HBsAg-positive cases were identified, and hepatitis B vaccination status was uncertain for 50% of patients.
Conclusion: Risk factor-based screening for HBV and HCV in primary care is a suboptimal approach. More than half of the patients testing positive lacked identifiable risk factors or elevated ALT levels. Universal one-time screening for all adults could address these limitations and significantly advance viral hepatitis elimination efforts.
Keywords: hepatitis B; hepatitis C; primary care; screening.
© 2025 The Author(s). Liver International published by John Wiley & Sons Ltd.
Conflict of interest statement
M.B. has served as a speaker and advisory board member for Gilead, Roche, and Arbutus. J.C.R.‐C. has served as a speaker for Gilead.
Figures
References
-
- Spearman W., Dusheiko G. M., Hellard M., and Sonderup M., “Hepatitis C,” Lancet 394 (2019): 1451. - PubMed
-
- Cooke G. S., Andrieux‐Meyer I., Applegate T. L., et al., “Accelerating the Elimination of Viral Hepatitis: A Lancet Gastroenterology & Hepatology Commission,” Lancet Gastroenterology & Hepatology 4, no. 2 (2019): 135–184. - PubMed
-
- World Health Organization , Combating Hepatitis B and C to Reach Elimination by 2030 (World Health Organ, 2016), http://apps.who.int/iris/bitstream/10665/206453/1/WHO_HIV_2016.
-
- World Health Organization , Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections (World Health Organization, 2021), https://www.who.int/publications/i/item/9789240027077.
-
- Blach S., Terrault N. A., Tacke F., et al., “Global Change in Hepatitis C Virus Prevalence and Cascade of Care Between 2015 and 2020: A Modelling Study,” Lancet Gastroenterology & Hepatology 7, no. 5 (2022): 396–415. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous