Glucagon-like peptide-1 (GLP-1) receptor agonists in inflammatory bowel disease: mechanisms, clinical implications, and therapeutic potential
- PMID: 40972535
- PMCID: PMC12558716
- DOI: 10.1093/ecco-jcc/jjaf167
Glucagon-like peptide-1 (GLP-1) receptor agonists in inflammatory bowel disease: mechanisms, clinical implications, and therapeutic potential
Abstract
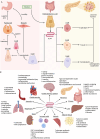
Glucagon-like peptide-1 receptor agonists are increasingly recognized for their potential dual benefit in inflammatory bowel disease (IBD), offering metabolic advantages alongside emerging anti-inflammatory, immunomodulatory, and gut barrier-enhancing effects. Pre-clinical data demonstrate attenuation of inflammation, preservation of epithelial integrity, and modulation of the microbiome in colitis models. Early retrospective studies in patients with IBD suggest improved clinical outcomes, such as reduced hospitalization and surgery rates, particularly in those with obesity. Glucagon-like peptide-1 receptor agonists are already widely used for obesity and diabetes, including increasing self-administration by patients outside medical supervision. Their impact on drug absorption, safety in gastrointestinal disease, and interactions with existing IBD therapies require further exploration. This review synthesizes the mechanistic rationale, pre-clinical evidence, and clinical data to date, highlighting the potential utility and safety considerations of glucagon-like peptide-1 receptor agonists in IBD and emphasizes the need for robust prospective trials to ascertain their safety and efficacy in this patient population.
Keywords: Crohn’s disease; glucagon-like peptide-1 receptor agonists; inflammatory bowel disease; ulcerative colitis.
© The Author(s) 2025. Published by Oxford University Press on behalf of European Crohn’s and Colitis Organisation.
Conflict of interest statement
M.C. served as a speaker and an advisory board member of or has received grants from Pfizer, Celltrion, Ferring, and Dr Falk. S.P.—None. R.P. has provided consultancy to Galapagos. K.P. has received honoraria for educational meetings and speaker fees from Abbvie, Janssen, Takeda, Dr Falk Pharma, PredictImmune, Pfizer, and Ferring and has received advisory board fees from Abbvie, Galapagos, Pfizer, and Janssen. He has also received a grant from Abbvie to support research. J.G.—None. T.A.—is supported by an NIHR Senior Clinical and Practitioner Award and reports institutional grants or contracts from Nova Pharmaceuticals, Astra-Zeneca; consulting fees from Amgen, Celltrion, Janssen, Eli Lilly; support for attending meetings and/or travel from Tillotts and Celltrion Healthcare. S.H. served as a speaker, a consultant, and an advisory board member or has received grants from Pfizer, Janssen, AbbVie, Takeda, Alfasigma, Ferring, Lilly, Pharmacosmos, and Banook Group.
Figures


References
-
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769-2778. - PubMed
-
- Ng M, Gakidou E, Lo J, et al. Global, regional, and national prevalence of adult overweight and obesity, 1990–2021, with forecasts to 2050: a forecasting study for the Global Burden of Disease Study 2021. The Lancet. 2025;405:813-838.
-
- Goldman Sachs. Why the anti-obesity drug market could grow to $100 billion by 2030 [Internet]. [cited 2025 Apr 13]; Available from: https://www.goldmansachs.com/insights/articles/anti-obesity-drug-market
-
- Wong HJ, Sim B, Teo YH, et al. Efficacy of GLP-1 receptor agonists on weight loss, BMI, and waist circumference for patients with obesity or overweight: a systematic review, meta-analysis, and meta-regression of 47 randomized controlled trials. Diabetes Care. 2025;48:e86-e87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

