Global, regional, and national burden of headache disorders, 1990-2021, with forecasts to 2050: A Global Burden of Disease study 2021
- PMID: 40972580
- PMCID: PMC12629824
- DOI: 10.1016/j.xcrm.2025.102348
Global, regional, and national burden of headache disorders, 1990-2021, with forecasts to 2050: A Global Burden of Disease study 2021
Abstract
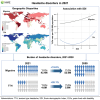
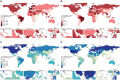
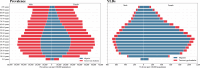
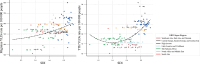
Headache disorders, especially migraines and tension-type headaches (TTHs), are major global public health concerns, as shown by the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2021. We provide updated global estimates of prevalence and years lived with disability (YLDs) from 1990 to 2021 across 204 countries and territories and forecasts through 2050. In 2021, there are 2.0 billion people with TTH and 1.2 billion with migraine. Although TTH is more prevalent, migraine causes higher disability. While crude prevalence and YLDs increased, age-standardized rates remained stable and are projected to continue this trend due to population growth. There is a disproportionately higher burden in women aged 30-44 and countries with higher Socio-demographic Index and Healthcare Access and Quality Index. Despite this, migraines remain underrecognized in health policies and funding. This study emphasizes the urgent need to prioritize headache disorders in global health agendas.
Keywords: Global Burden of Disease; disability-adjusted life years; headache disorders; migraine; tension-type headache.
Copyright © 2025 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests S. Ashina reports royalties or licenses from McGraw Hill/Medical; consulting fees from Lundbeck, Eli Lilly, Pfizer, AbbVie, Satsuma, Linpharma, Theranica, and Impel NeuroPharma; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Lundbeck, Teva, Pfizer, AbbVie, and Eli Lilly; and leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid from the Board Trustee, International Headache Society and as a Member of Education Committee, International Headache Society; other financial or non-financial interests as Associate Editor of Cephalalgia, Associate Editor of Frontiers in Neurology, and Associate Editor of BMC Neurology; outside the submitted work. S. Bhaskar reports grants or contracts from Japan Society for the Promotion of Science (JSPS); Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT); Grant-in-Aid for Scientific Research (KAKENHI) (grant ID: 23KF0126); JSPS and the Australian Academy of Science; and JSPS International Fellowship (grant ID: P23712). S. Bhaskar reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid as District Chair, Diversity, Equity, Inclusion & Belonging of Rotary District 9675 (Sydney, Australia); as Chair, Founding Member and Manager of the Global Health & Migration Hub Community, Global Health Hub Germany (Berlin, Germany); as Editorial Board Member of PLOS One, BMC Neurology, Frontiers in Neurology, Frontiers in Stroke, Frontiers in Public Health, Journal of Aging Research, Neurology International, Diagnostics, & BMC Medical Research Methodology; as a member of the College of Reviewers, Canadian Institutes of Health Research (CIHR), Government of Canada; as the Director of Research of World Headache Society (Bengaluru, India); as Expert Adviser/Reviewer of Cariplo Foundation (Milan, Italy); as Visiting Director of National Cerebral and Cardiovascular Center, Department of Neurology, Division of Cerebrovascular Medicine and Neurology, Suita (Osaka, Japan); as Member, Scientific Review Committee of Cardiff University Biobank (Cardiff, UK); as Chair of Rotary Reconciliation Action Plan; and as Healthcare and Medical Adviser at Japan Connect (Osaka, Japan) outside the submitted work. A. Biswas reports consulting fees from Intas Pharmaceuticals, Lupin Pharmaceuticals, Alkem Laboratories, and Torrent Pharmaceuticals, outside the submitted work. C.E. reports grants or contracts from Río Hortega grant Acción Estratégica en Salud 2017–2020 from Instituto de Salud Carlos III (CM20/00217) and the Juan Rodés fellowship, Subprograma Estatal de Incorporación de la Acción Estratégica en Salud 2023 (JR23/00065) from Instituto de Salud Carlos III; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Organon, TEVA, Lilly, Dr. Reddy’s, and Novartis; and support for attending meetings and/or travel from TEVA and Lundbeck outside the submitted work. X.D. reports support for the present manuscript from the Institute for Health Metrics and Evaluation, University of Washington. A Dima reports other financial interests with the Bucharest University of Economic Studies (Romania), outside the submitted work. A. Faro reports support for the present manuscript from the National Council for Scientific and Technological Development (CNPq, Brazil), personal grant “Researcher at CNPq - Level 1B.” E.G.F. reports payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Biogen, Novartis, Merck, Roche, and Astra Zeneca and support for attending meetings and/or travel from Biogen, Novartis, Merck, Roche, and Astra Zeneca outside the submitted work. A.H. reports consulting fees from Novartis, Sanofi Genzyme, Biologix, Merck, Hikma Pharma, Janssen, Inspire Pharma, Future Pharma, and Elixir pharma; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Novartis, Allergan, Merck, Biologix, Janssen, Roche, Sanofi Genzyme, Bayer, Hikma Pharma, Al Andalus, Chemipharm, Lundbeck, Inspire Pharma, Future Pharma and Habib Scientific Office, and Everpharma; and support for attending meetings and/or travel from Novartis, Allergan, Merck, Biologix, Roche, Sanofi Genzyme, Bayer, Hikma Pharma, Chemipharm, and Al Andalus and Clavita pharm. A.H. reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid as the Vice President of MENA headache society; Board member of Multiple Sclerosis chapter of the Egyptian Society of Neurology; Board member of head-ache chapter of the Egyptian Society of Neurology; and member of committee of Education of the international Headache Society (IHS), membership committee of IHS, and regional committee of HIS outside the submitted work. I.I. reports support for the present manuscript from the Ministry of Science, Technological Development and Innovation of the Republic of Serbia (grant no. 451-03-137/2025-03/200110). N.E.I. reports leadership or fiduciary role in other board, society, committee or advocacy group, unpaid as Bursar and Council Member of the Malaysian Academy of Pharmacy (Malaysia) and committee member of Malaysian Pharmacists Society Education Chapter Committee outside the submitted work. K. Krishan reports other, non-financial support from the UGC Centre of Advanced Study, CAS II, awarded to the Department of Anthropology, Panjab University (Chandigarh, India), outside the submitted work. M.L. reports grants or contracts from the Italian Ministry of Health for research on disorders of consciousness as well as grants from EU on NCDs and CVDs impact and support for attending meetings and/or travel as Board member of the European Academy of Neurology and of the Italian Society of neurology outside the submitted work. S. Mohammed reports support for the present manuscript from the Gates Foundation. L.M. reports support for the present manuscript from the Italian Ministry of Health (Ricerca Corrente 34/2017), payments made to the Institute for Maternal and Child Health IRCCS Burlo Garofolo. W.O. reports grants or contracts from Pfizer, Libbs, Teva, Ease Labs, and Mantecorp; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Pfrizer, Libbs, Teva, Ease Labs, and Mantecorp; support for attending meetings and/or travel from Mantecorp; leadership or fiduciary role in other board, society, committee, or advocacy group, unpaid with the Public Policies Committee (Brazilian Headache Society) outside the submitted work. M.F.P.P. reports grants or contracts from Abbvie and Pfizer; consulting fees from Abbvie, Pfizer, and Teva; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Abbvie, Pfizer, and Teva; patents planned, issued, or pending (US 17/196,611); leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid with Abraces and IHS outside the submitted work. M.A.P. reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid as editor in chief of Annals of Clinical and Experimental Neurology outside the submitted work. P.P.-R. reports grants or contracts from ERANet Neuron, Instituto Salud Carlos III, and Novartis; consulting fees from AbbVie, Almirall, Dr. Reddy’s, Eli Lilly, Lundbeck, Organon, Pfizer, and Teva; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from AbbVie, Almirall, Dr. Reddy’s, Eli Lilly, Lundbeck, Novartis, Organon, Pfizer, and Teva; support for attending meetings and/or travel from Lundbeck, Organon, and Teva; and leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid with the Honorary Secretary International Headache Society outside the submitted work. J.I.S. reports other financial or non-financial support from the Yonsei Fellowship. J.S. reports consulting fees from ROMTech, Atheneum, Clearview healthcare partners, American College of Rheumatology, Yale, Hulio, Horizon Pharmaceuticals, DINO-RA, ANI/Exeltis, USA Inc., Frictionless Solutions, Schipher, Crealta/Horizon, Medisys, Fidia, PK Med, Two labs Inc., Adept Field Solutions, Clinical Care options, Putnam associates, Focus forward, Navigant consulting, Spherix, MedIQ, Jupiter Life Science, UBM LLC, Trio Health, Medscape, WebMD, and Practice Point communications, and the National Institutes of Health; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events on the speaker’s bureau of Simply Speaking; support for attending meetings and/or travel from the speaker’s bureau of Simply Speaking; leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid as a Past steering committee member of the OMERACT, an international organization that develops measures for clinical trials and receives arm’s length funding from 12 pharmaceutical companies, as the Chair of the Veterans Affairs Rheumatology Field Advisory Committee, and as the editor and the Director of the UAB Cochrane Musculoskeletal Group Satellite Center on Network Meta-analysis; and stock or stock options in Atai life sciences, Kintara therapeutics, Intelligent Biosolutions, Acumen pharmaceutical, TPT Global Tech, Vaxart pharmaceuticals, Atyu biopharma, Adaptimmune Therapeutics, GeoVax Labs, Pieris Pharmaceuticals, Enzolytics Inc., Seres Therapeutics, Tonix Pharmaceuticals Holding Corp., Aebona Pharmaceuticals, and Charlotte’s Web Holdings, Inc., and previously owned stock options in Amarin, Viking, and Moderna pharmaceuticals outside the submitted work. M. Tanwar reports grants or contracts from the University of Alabama at Birmingham Health Service Foundation grant for TBI research outside the submitted work. M.Z. reports other financial support as an Alexion, AstraZeneca Rare Disease employee, outside the submitted work.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

