Accuracy of glomerular filtration rate estimates among patients with cancer
- PMID: 40973716
- PMCID: PMC12644916
- DOI: 10.1038/s41416-025-03190-3
Accuracy of glomerular filtration rate estimates among patients with cancer
Abstract
Background: Glomerular filtration rate (GFR) estimation is a key issue in determining cancer treatment eligibility and dosing of treatments with narrow therapeutic index. Yet, little is known about the accuracy of GFR estimation among people with cancer in routine care.
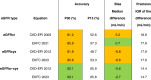
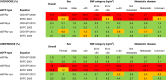
Methods: In a cross-sectional study including 1611 adults with cancer referred for 1837 determinations of measured GFR (mGFR), we assessed the accuracy of estimated GFR based on creatinine (eGFRcr), cystatin C (eGFRcys) and their combination (eGFRcr-cys). Accuracy was reported as percentage of patients with estimated values within 30% of mGFR; bias and precision as the median and interquartile range of eGFR-mGFR, respectively. Dosing accuracy was assessed by calculating expected dose of carboplatin for area under the curve of 5 mg/mL/min using the Calvert formula.
Results: Median age was 68 (IQI 61 to 74) years, 38.5% were female with mean mGFR 75 (SD 30) mL/min; 17% had metastatic disease. Accuracy, bias and precision were best for eGFRcr-cys. Using eGFRcr would recommend an "overdose" of carboplatin in 10-20% of participants: this was 3-4 times less common using eGFRcr-cys.
Conclusion: eGFRcr-cys equations provide the most accurate estimates of mGFR in patients with cancer, with potential to improve dosing accuracy substantially compared to eGFRcr.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Outside the submitted work, J.S.L. has received personal consultancy fees from Boehringer Ingelheim and lectureship honoraria from Astra Zeneca. Outside the submitted work, P.B.M. has received honoraria for lectures and advisory boards from AstraZeneca, Vifor, Pharmacosmos, GSK, Bayer and Boehringer Ingelheim and research funding from AstraZeneca and Boehringer Ingelheim. Outside of the submitted work, B.M.P.E. has received honoraria for lectures from AstraZeneca. R.J.J. has personally received honoraria for lectures and consultancy fees from Astellas, AstraZeneca, Bayer, Bristol Myers Squibb, Johnson and Johnson, Merck KGaA, Merck Sharp Dohme, Novartis, Pfizer, and Roche, and research funding from Astellas, AstraZeneca, Clovis, Exelixis and Pfizer. L.A.I. reports financial support paid to Tufts Medical Center from the National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases, National Kidney Foundation, Chinnocks, Alexion and Astra Zeneca; and participation on the medical advisory council for Alport Foundation and the scientific advisory board for National Kidney Foundation. J.J.C. reports grants paid to Karolinska Institutet from AstraZeneca, Boehringer Ingelheim, CSL Vifor, MSD, Astellas, NovoNordisk, all outside the submitted work; personal fees for lectures or advisory board meetings from Fresenius Kabi, and Laboratorios Columbia, all outside the submitted work. Ethics approval and consent to participate: This study was conducted in accordance with Strengthening The Reporting of OBservational studies in Epidemiology (STROBE) guidance. SCREAM was approved by the Stockholm Ethics Review Board with a waiver of consent (reference 2017/793-31); informed consent was not deemed necessary because all data were deidentified at the Swedish Board of Health and Welfare.
Figures

References
-
- Krens SD, Lassche G, Jansman FGA, Desar IME, Lankheet NAG, Burger DM, et al. Dose recommendations for anticancer drugs in patients with renal or hepatic impairment. Lancet Oncol. 2019;20:e200–7. - PubMed
-
- Costa E Silva VT, Gil LA, Inker LA, Caires RA, Costalonga E, Coura-Filho G, et al. A prospective cross-sectional study estimated glomerular filtration rate from creatinine and cystatin C in adults with solid tumors. Kidney Int. 2022;101:607–14. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

