Clonal evolution and apoptosis resistance in myelodysplastic neoplasms and acute myeloid leukemia under treatment: insights from integrative longitudinal profiling
- PMID: 40973765
- PMCID: PMC12634422
- DOI: 10.1038/s41375-025-02756-7
Clonal evolution and apoptosis resistance in myelodysplastic neoplasms and acute myeloid leukemia under treatment: insights from integrative longitudinal profiling
Abstract
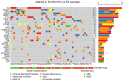
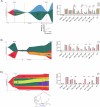
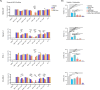
Treatment of high-risk Myelodysplastic Neoplasms (hr-MDS) and (secondary) Acute Myeloid Leukemia (AML) remains a clinical challenge. The combination of azacitidine and venetoclax (aza/ven) may improve treatment outcomes, but still fails in a significant fraction of patients. We established a single-center collection of longitudinal samples from patients with MDS and AML/sAML and performed comprehensive genetic, proteomic and functional apoptosis profiling to identify biomarkers and targetable escape mechanisms to aza/ven. Baseline genetic characterization (n = 55) identified high-risk genetic alterations, while longitudinal analyses (n = 268, mean 8.7 [3-20] timepoints) revealed distinct genetic profiles of clonal evolution. Functional BH3-profiling at treatment initiation identified heterogeneous dependencies on BCL-2 family members. Notably, high BCL-2 dependence correlated with genetic response to aza/ven and improved overall survival, whereas increased BCL-xL dependence was associated with resistance. We further identified patterns of acquired resistance, with loss of apoptotic priming and shifts in anti-apoptotic dependencies contributing to treatment failure. BH3 profiling revealed functional shifts toward MCL-1 and/or BCL-xL in individual cases, suggesting potential therapeutic targets to overcome resistance. In vitro, BCL-xL inhibition effectively counteracted resistance in increased BCL-xL dependence cases. In summary, we characterized treatment-associated clonal evolution in MDS and AML, providing insights into clinical response, disease progression and potential individualized therapeutic strategies.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: DH: Advisory board, research support and honoraria by Bristol Myers Squibb and JAZZ. ES received honoraria from Amgen, BMS, Stemline, Sanofi, Incyte, Janssen, Takeda and JAZZ. RK received honoraria from Takeda, Janssen and Novartis. All other authors declare no conflict of interest. Ethics approval and consent to participate: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local Ethics Committee of the University Medical Center Göttingen (application number 02-02-14). Informed consent was obtained from all patients.
Figures





References
-
- Raza A, Gezer S, Mundle S, Gao XZ, Alvi S, Borok R, et al. Apoptosis in bone marrow biopsy samples involving stromal and hematopoietic cells in 50 patients with myelodysplastic syndromes. Blood. 1995;86:268–76. - PubMed
-
- Janssen J, Buschle M, Layton M, Drexler H, Lyons J, van den Berghe H, et al. Clonal analysis of myelodysplastic syndromes: evidence of multipotent stem cell origin. Blood. 1989;73:248–54. - PubMed
-
- Tefferi A, Vardiman JW. Myelodysplastic syndromes. N Engl J Med. 2009;19:1872–85. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

