The invasion pore induced by Toxoplasma gondii
- PMID: 40973829
- PMCID: PMC12549902
- DOI: 10.1038/s44319-025-00565-8
The invasion pore induced by Toxoplasma gondii
Abstract
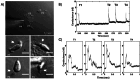
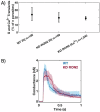
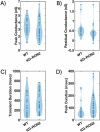
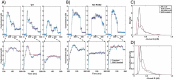
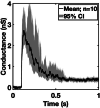
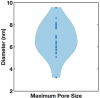
The parasite Toxoplasma gondii invades its host cell only after secreting proteins such as invasion-requisite RON2 that inserts into the host cell membrane to establish the moving junction. Electrophysiological recordings at sub-200 µs resolution show a transient increase in host cell membrane conductance following parasite exposure. Transients always precede invasion, but parasites depleted of RON2 generate transients without invading. Thus RON2 is not essential for transient generation. Time-series analysis developed here and applied to the 910,000 data point transient dataset reveal multiple quantal conductance changes in the parasite-induced transient, consistent with rapid insertion, then slower removal, blocking, or inactivation of potential pore components. Quantal steps for wild-type RH strain parasites have a principal mode with Gaussian mean of 0.26 nS, similar in step size to the pore forming protein EXP2, part of the PTEX translocon of malaria parasites. Without RON2 the quantal mean (0.19 nS) is significantly different. Because we observe no parasite invasion without poration, the term "invasion pore" is proposed to describe this transient breach in host cell membrane barrier integrity during invasion.
Keywords: Cell Membrane; Membrane Poration; Parasite; Rhoptry Secretion; Whole-Cell Patch-Clamp.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures





Update of
-
The invasion pore induced by Toxoplasma gondii.bioRxiv [Preprint]. 2024 Nov 28:2024.10.11.617945. doi: 10.1101/2024.10.11.617945. bioRxiv. 2024. Update in: EMBO Rep. 2025 Oct;26(20):5009-5026. doi: 10.1038/s44319-025-00565-8. PMID: 39416144 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

