Drivers of hospital costs in ANCA-associated vasculitis patients with long-term follow-up-a real-world cost analysis
- PMID: 40978114
- PMCID: PMC12448927
- DOI: 10.1093/ckj/sfaf267
Drivers of hospital costs in ANCA-associated vasculitis patients with long-term follow-up-a real-world cost analysis
Abstract
Background: Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a potentially life-threatening, systemic autoimmune disease with a high risk for relapse and treatment-related toxicity, making AAV a high-costs illness. This study aimed to identify clinical insights for clinicians on considering this costs burden.
Methods: We conducted a detailed, retrospective, single-centre, activity-based cost-analysis and identified clinical variables associated with increased costs. We analysed real-world costs incurred by the hospital between January 2018 and December 2019, omitting the outpatient pharmacy expenditures. Our cohort included both incident and prevalent AAV patients with at least 6 months of follow-up since diagnosis, indicating survival beyond initial diagnosis.
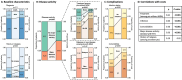
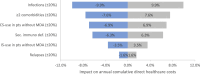
Results: For 180 AAV patients with a median follow-up of 1.8 years the average hospital costs incurred amounted to €9887 per patient year, with inpatient care being the primary cost driver (32%). Merely 15% of costs were attributable to patients experiencing relapse (N = 14/180, 8%). More importantly, 71% of costs were attributable to patients experiencing infections (N = 77/180, 43%). Likewise, 60% of costs were attributable to patients with multi-comorbidity (N = 65/180, 36%). Infections and multi-comorbidity were both strongly associated with corticosteroid (CS) use. Regression and sensitivity analyses suggest that a reduction of infections, comorbidities and maintenance treatment with CS will reduce hospital costs.
Conclusion: This real-world cost analysis demonstrates that the burden of infections and comorbidities, both related to CS use, is higher than that of relapses on hospital costs in AAV patients. Thus, this study implicates clinicians considering hospital costs should focus on reducing CS and achieving CS-free remission to prevent infections and comorbidities.
Keywords: ANCA-associated vasculitis; corticosteroids; healthcare costs; pauci-immune glomerulonephritis; systemic autoimmune disease.
© The Author(s) 2025. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
The work of Y.K.O.T. is supported by the Arthritis Research and Collaboration Hub (ARCH) foundation. ARCH is funded by Dutch Arthritis Foundation (ReumaNederland). The LUMC received an unrestricted research grant from GlaxoSmithKline, Aurinia Pharmaceuticals and Vifor Pharma for investigator-initiated studies conducted by Y.K.O.T. The LUMC received consulting fees from Aurinia Pharmaceuticals, Novartis, GSK, KezarBio, Vifor Pharma, Otsuka Pharmaceuticals on consultancies delivered by Y.K.O.T.
Figures



References
-
- Hellmich B, Sanchez-Alamo B, Schirmer JH et al. EULAR recommendations for the management of ANCA-associated vasculitis: 2022 update. Ann Rheum Dis 2024;83:30–47. - PubMed
-
- Kidney Disease: Improving Global Outcomes (KDIGO) ANCA Vasculitis Work Group. KDIGO 2024 Clinical Practice Guideline for the Management of Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Vasculitis. Kidney Int 2024;105:S71–116. - PubMed
-
- Patel NJ, Jayne DRW, Merkel PA et al. Glucocorticoid Toxicity Index scores by domain in patients with antineutrophil cytoplasmic antibody-associated vasculitis treated with avacopan versus standard prednisone taper: post-hoc analysis of data from the ADVOCATE trial. Lancet Rheumatol 2023;5:e130–8. 10.1016/S2665-9913(23)00030-9 - DOI - PubMed
LinkOut - more resources
Full Text Sources

