Design, Synthesis, and Trypanosomicidal Evaluation of Eugenol-Based Azole Hybrids: Discovery of an In Vitro Active and Selective Compound
- PMID: 40978380
- PMCID: PMC12444550
- DOI: 10.1021/acsomega.5c06619
Design, Synthesis, and Trypanosomicidal Evaluation of Eugenol-Based Azole Hybrids: Discovery of an In Vitro Active and Selective Compound
Abstract
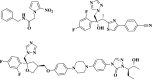
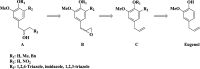
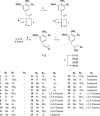
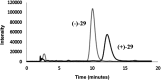
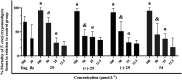
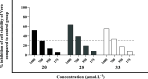
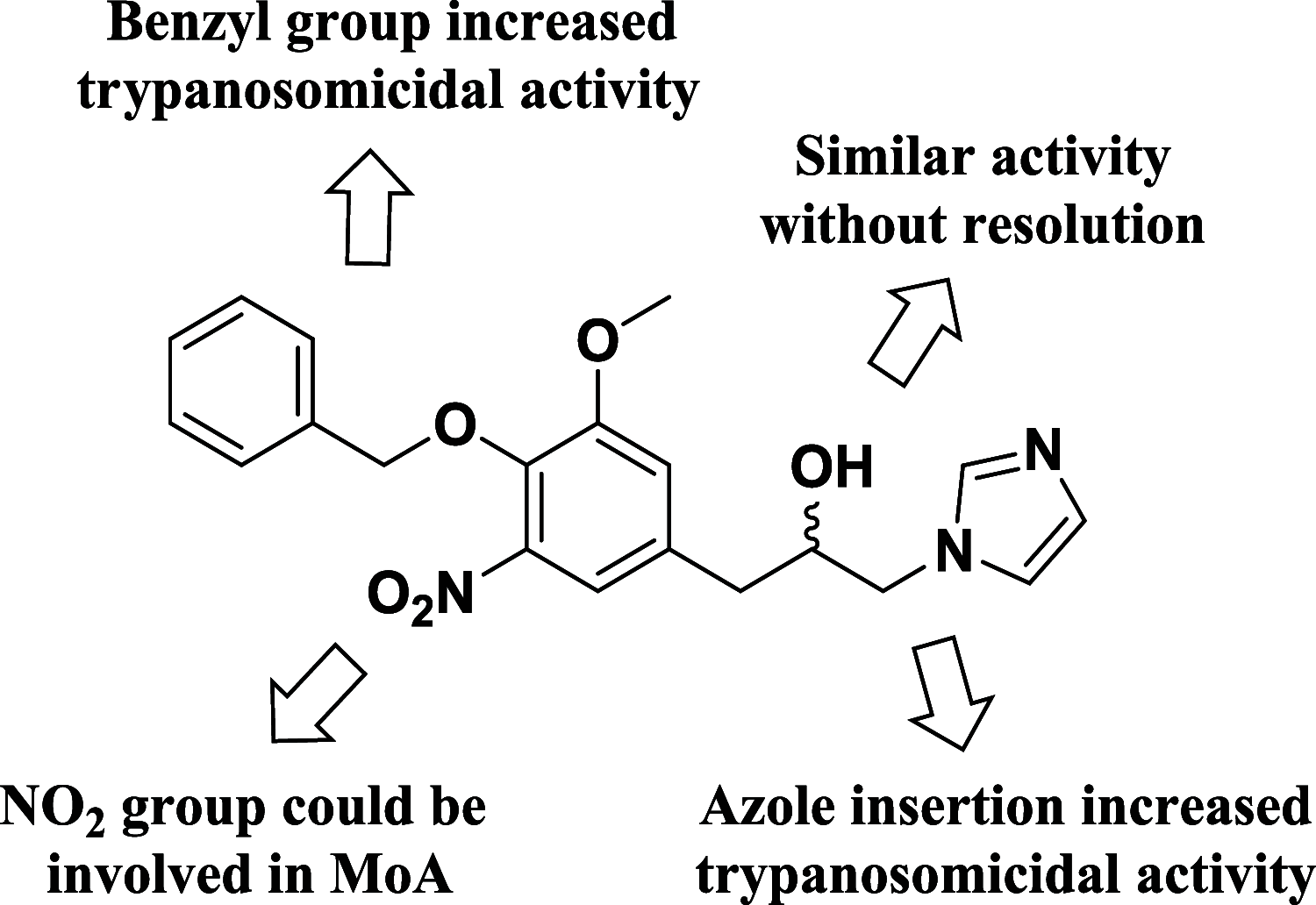
Chagas disease, caused by Trypanosoma cruzi, remains a major public health concern in Latin America and beyond, with limited treatment options and high morbidity in chronic stages. Currently, benznidazole remains the first-line therapy for Chagas disease but exhibits considerable limitations, including dose-dependent toxicity and poor efficacy in long-term infections. In response to the pressing need for safer and more effective therapies, this study reports the design, synthesis, and biological evaluation of 17 novel eugenol-based hybrid compounds (18-34), strategically constructed by integrating pharmacophoric features from nitroaromatic trypanosomicidal agents, azole-based CYP51 inhibitors, and the phenylpropanoid core of eugenol. The synthetic sequence included key steps such as nitration, O-alkylation, epoxidation, nucleophilic epoxide opening, and CuAAC reactions to afford 1,2,4-triazole-, imidazole-, and 1,2,3-triazole-containing hybrids. All compounds were structurally characterized by IR, HRMS, and NMR spectroscopy. Biological assays were performed against both trypomastigote and amastigote forms of T. cruzi (Y strain), revealing compound-dependent antiparasitic profiles. Hybrid 29, bearing benzyl and nitro substituents on an imidazole ring, emerged as the most active candidate (EC50 = 26.5 μmol·L-1) with a selectivity index exceeding 49. Enantioseparation by chiral HPLC enabled the isolation of (+)-29 and (-)-29 enantiomers, both of which showed comparable activity, indicating no significant stereoselectivity. Cytotoxicity assays in mammalian cell lines (Vero and H9c2) confirmed low toxicity for most hybrids (CC50 > 1300 μmol·L-1). Although the compounds were inactive against intracellular amastigotes, the observed trypomastigote-selective activityparticularly of nitrobenzylated derivatives suggests a non-CYP51-related mechanism of action. These findings highlight the value of phenylpropanoid-based molecular hybridization as a promising strategy for developing new antiparasitic agents against T. cruzi.
© 2025 The Authors. Published by American Chemical Society.
Figures

References
-
- Santoro G. F., Cardoso M. G., Guimarães L. G., Mendonça L. Z., Soares M. J.. Trypanosoma cruzi: activity of essential oils from Achillea millefolium L., Syzygium aromaticum L. and Ocimum basilicum L. on epimastigotes and trypomastigotes. Exp. Parasitol. 2007;116(3):283–290. doi: 10.1016/j.exppara.2007.01.018. - DOI - PubMed
-
- Marchese A., Barbieri R., Coppo E., Orhan I. E., Daglia M., Nabavi S. F., Izadi M., Abdollahi M., Nabavi S. M., Ajami M.. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017;43(6):668–689. doi: 10.1080/1040841X.2017.1295225. - DOI - PubMed
LinkOut - more resources
Full Text Sources
