Removal of the Metal Ion Lead II from Aqueous Effluents Using the Stem Bark of Ximenia americana L
- PMID: 40978452
- PMCID: PMC12444525
- DOI: 10.1021/acsomega.5c01460
Removal of the Metal Ion Lead II from Aqueous Effluents Using the Stem Bark of Ximenia americana L
Abstract
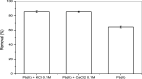
In recent years, the pollution and contamination of water with heavy metals have posed significant risks to human health and have compromised aquatic life. As a result, the use of biosorbent materials to remove these metals from effluents has emerged as an alternative method for their remediation. This study evaluated the adsorption potential of Ximenia americana L. stem bark (in natura) for the removal of Pb2+ from aqueous solutions. The process involved the collection, washing, drying, and grinding of the shell, resulting in a powder used for characterization techniques, kinetic assays (conducted at intervals from 1 to 120 min), isotherms using metal solutions ranging from 40 to 600 mg L-1, and adsorption thermodynamics at temperatures of 278.15, 298.15, and 318.15 K. Desorption assays showed that the adsorbent is reusable, maintaining its efficiency in the adsorption process without significant losses. In coexisting ion tests, it was observed that the evaluated ions did not compete for the same active sites, indicating selectivity in adsorption. The point of zero charge (PZC) of the material was determined to be 6.0, suggesting that its surface becomes positively charged at pH values below this point, favoring the adsorption of negatively charged species. In dosage tests, the amount of material used did not directly influence the adsorption process. The material was characterized using scanning electron microscopy (SEM), which revealed significant morphological differences among the bark samples, including variations in particle shape and size; these differences may affect adsorption capacity. After treatment with Pb2+ ions, crystals were observed on the surface of the bark, suggesting the formation of complexes between the metal ions and biomass components, potentially increasing the material's affinity for the metal. Fourier-transform infrared spectroscopy (FT-IR) showed changes in absorbance bands, highlighting the presence of compounds such as aromatics, carbonyls, and esters. X-ray fluorescence (XRF) analysis revealed a significant difference in Pb2+ ion retention between treated (13.12%) and untreated bark (0.0%). The fact that the raw shells contained no residual metals indicates a promising potential for metal ion removal processes without the need for additional chemical treatments. The adsorption kinetics followed a pseudo-second-order kinetic model, with an adjusted R 2 of 0.999. Equilibrium adsorption data were analyzed and best fitted the Sips adsorption model, with a q max of 138.20 mg g-1, and the best fit achieved at 278.15 K with an R 2 value of 0.998. This indicates that the adsorption process is based on chemical interactions, demonstrating high efficiency in capturing Pb2+ and highlighting the strong affinity of the material for the metal ion, as well as its potential for environmental decontamination. Thermodynamic analysis showed that the reaction was spontaneous at all studied temperatures, with ΔG° values of -3.21, -9.01, and -14.8 kJ mol-1. The process was endothermic, with ΔH° = 77.4 kJ mol-1, and showed increased system disorder, as indicated by ΔS° = 0.29 kJ mol-1 K-1. In view of these findings, this study contributes to the development of sustainable alternatives for environmental decontamination and highlights X. americana as a promising natural resource for the treatment of wastewater and industrial effluents, its bark, although little explored, presents high efficiency, low cost, selectivity and reuse, characteristics that position this biosorbent as a viable alternative, compared to conventional technologies such as activated carbon, membranes and nanomaterials.
© 2025 The Authors. Published by American Chemical Society.
Figures











References
-
- Peng Y., Chen R., Yang R.. Analysis of heavy metals in Pseudostellaria heterophylla in Baiyi Country of Wudang District. J. Geochem. Explor. 2017;176:57–63. doi: 10.1016/j.gexplo.2016.02.011. - DOI
-
- Moreira E. M. S., Silva J. D. P.. Gestão de Resíduos Líquidos e Sólidos Gerados em Processos de Laticínios: Provocações Para o Engenheiro Químico. Rev. Foco. 2024;17(10):e6540. doi: 10.54751/revistafoco.v17n10-093. - DOI
-
- Rios B., Batista P. F. da S., Crystello D. C. B.. Impactos Da poluição E alteração De Habitat Em Ecossistemas De água Doce: Uma revisão bibliográfica. Cad. Pedagógico. 2024;21:e6568. doi: 10.54033/cadpedv21n8-086. - DOI
-
- Aguiar M. R. M. P. d., Novaes A. C., Guarino A. W. S.. Remoção de metais pesados de efluentes industriais por aluminossilicatos. Quím. Nova. 2002;25(6b):1145–1154. doi: 10.1590/s0100-40422002000700015. - DOI
-
- Alloway, B. J. ; Ayres, D. C. . Chemical Principles of Environmental Pollution, 2th ed: Blackie Academic & Professional, 1997. United Kindon.
LinkOut - more resources
Full Text Sources
Research Materials
