Cohort event monitoring of safety of COVID-19 vaccines: the Italian experience of the "ilmiovaccinoCOVID19 collaborating group"
- PMID: 40979378
- PMCID: PMC12445166
- DOI: 10.3389/fdsfr.2024.1363086
Cohort event monitoring of safety of COVID-19 vaccines: the Italian experience of the "ilmiovaccinoCOVID19 collaborating group"
Abstract
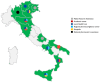
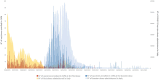
Introduction: In 2021, the European Medicines Agency supported the "Covid Vaccine Monitor (CVM)," an active surveillance project spanning 13 European countries aimed at monitoring the safety of COVID-19 vaccines in general and special populations (i.e., pregnant/breastfeeding women, children/adolescents, immunocompromised people, and people with a history of allergies or previous SARS-CoV-2 infection). Italy participated in this project as a large multidisciplinary network called the "ilmiovaccinoCOVID19 collaborating group." Methods: The study aimed to describe the experience of the Italian network "ilmiovaccinoCOVID19 collaborating group" in the CVM context from June 2021 to February 2023. Comprising about 30 partners, the network aimed to facilitate vaccinee recruitment. Participants completed baseline and follow-up questionnaires within 48 h from vaccination over a 6-month period. Analyses focused on those who completed both the baseline and the first follow-up questionnaire (Q1), exploring temporal trends, vaccination campaign correlation, and loss to follow-up. Characteristics of recruited vaccinees and vaccinee-reported adverse drug reactions (ADRs) were compared with passive surveillance data in Italy. Results: From June 2021 to November 2022, 22,384,663 first doses and 38,207,452 booster doses of COVID-19 vaccines were administered in Italy. Simultaneously, the study enrolled 1,229 and 2,707 participants for the first and booster doses, respectively. Of these, 829 and 1,879 vaccinees, respectively, completed both baseline and at least Q1 and were included in the analyses, with a significant proportion of them (57.8%/34.3%) belonging to special cohorts. Most vaccinees included in the analyses were women. Comirnaty® (69%) and Spikevax® (29%) were the most frequently administered vaccines. ADR rates following Comirnaty® and Spikevax® were higher after the second dose, particularly following Spikevax®. Serious ADRs were infrequent. Differences were observed in ADR characteristics between CVM and Italian passive surveillance. Conclusion: This study confirmed the favorable safety profile of COVID-19 vaccines, with findings consistent with pivotal clinical trials of COVID-19 vaccines, although different proportions of serious ADRs compared to spontaneous reporting were observed. Continuous evaluation through cohort event monitoring studies provides real-time insights crucial for regulatory responses. Strengthening infrastructure and implementing early monitoring strategies are essential to enhance vaccine safety assessment and prepare for future pandemics.
Keywords: COVID-19; active surveillance; passive surveillance; special cohorts; vaccine safety.
Copyright © 2024 Luxi, Bellitto, Ciccimarra, Cappello, L’Abbate, Bonaso, Ajolfi, Baldo, Bonaiuti, Costantino, De Sarro, Di Mauro, Fava, Ferri, Firenze, Furci, Gallelli, Leonardi, Negri, Pieraccini, Poluzzi, Sacripanti, Sangiorgi, Sapigni, Senesi, Tessari, Trabace, Vannacci, Venturini, Vitale, Zodda, Tuccori and Trifirò.
Conflict of interest statement
GT has served in the last 3 years on advisory boards/seminars funded by SANOFI, Eli Lilly, AstraZeneca, Abbvie, Servier, Mylan, Gilead, and Amgen; he was the scientific director of a master’s program on pharmacovigilance, pharmacoepidemiology, and real-world evidence, which has received a non-conditional grant from various pharmaceutical companies; he coordinated a pharmacoepidemiology team at the University of Messina until October 2020, which has received funding for conducting observational studies from various pharmaceutical companies (Boehringer Ingelheim, Daiichi Sankyo, and PTC Pharmaceuticals). He is also the scientific coordinator of the academic spin-off “INSPIRE srl,” which has received funding for conducting observational studies from contract research organizations (RTI Health Solutions and Pharmo Institute N.V.). None of these listed activities is related to the topic of the manuscript. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures




References
-
- Ahmadizar F., Luxi N., Raethke M., Schmikli S., Riefolo F., Saraswati P. W., et al. (2023). Safety of COVID-19 vaccines among the Paediatric population: analysis of the European surveillance systems and pivotal clinical trials. Drug Saf. 46 (6), 575–585. 10.1007/s40264-023-01304-5 - DOI - PMC - PubMed
-
- AIFA (2023a). COVID-19 vaccine surveillance report 14. Available at: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_va... (Accessed December 29, 2023).
-
- AIFA (2023b). COVID-19 vaccine surveillance report 3. Available at: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_va... (Accessed December 29, 2023).
-
- AIFA (2023c). COVID-19 vaccine surveillance report 8. Available at: https://www.aifa.gov.it/documents/20142/1315190/Rapporto_sorveglianza_va... (Accessed December 29, 2023).
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

