Relative exchangeable copper: A highly specific and sensitive biomarker for Wilson disease diagnosis
- PMID: 40980162
- PMCID: PMC12446559
- DOI: 10.1016/j.jhepr.2025.101537
Relative exchangeable copper: A highly specific and sensitive biomarker for Wilson disease diagnosis
Abstract
Background & aims: Wilson disease (WD) is an autosomal recessive disorder characterized by copper accumulation in various organs, primarily the liver and brain. Standard assessment of copper metabolism includes total serum copper, serum ceruloplasmin, and urinary copper excretion. Quantitative measurement of non-ceruloplasmin-bound copper, known as exchangeable copper (CuEXC), was developed in 2009. Subsequently in 2011, relative exchangeable copper (REC), defined as the ratio of CuEXC to total serum copper, was proposed as a diagnostic biomarker. This study aimed to validate the REC cut-off for the diagnosis of WD in a large cohort and to refine the reference ranges for CuEXC.
Methods: Data were collected from 778 individuals at the French National Reference Centre for WD from January 2009 to 2025. The cohort included 204 patients with WD, 359 healthy heterozygous carriers, and 215 controls. All participants underwent clinical evaluation, assessment of copper metabolism, including CuEXC and REC, and genetic testing for ATP7B. Receiver operating characteristic curve analysis was used to assess the diagnostic performance of REC and to determine the optimal cut-off for diagnosing WD.
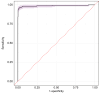
Results: Patients with WD had significantly higher CuEXC and REC values compared with heterozygous carriers and controls. The optimal REC cut-off for diagnosing WD was identified as 14% with 95.6% sensitivity and 99.8% specificity. This cut-off was validated in both pediatric and adult subgroups with similar sensitivity and specificity. Reference ranges for CuEXC (0.50-1.38 μmol/L) and for REC (2.6-9.5%) were refined using control group data. Age-specific ranges were also determined.
Conclusion: This study supports the use of REC in clinical practice and confirms its central role in the diagnostic algorithm for WD, as recognized in the recently published EASL 2025 guidelines.
Impact and implications: The study established relative exchangeable copper (REC) as a robust diagnostic biomarker for Wilson disease (WD), demonstrating high sensitivity and specificity across age groups in a large cohort including 204 patients with WD. By refining the optimal REC cut-off, this research provides crucial insights for improving WD diagnostic accuracy and patient outcomes. These findings confirm the need to incorporate REC into routine clinical practice and WD management guidelines, potentially reducing the reliance on invasive liver biopsies to assess hepatic copper levels. Consequently, this advancement in diagnostic methodology could facilitate earlier detection and treatment, thereby preventing irreversible tissue damage and enhancing the quality of life for patients with WD.
Keywords: Exchangeable copper; Hepatolenticular degeneration; Metabolic disease; Non-ceruloplasmin-bound copper (NCC); Relative exchangeable copper; Wilson disease.
© 2025 The Authors.
Conflict of interest statement
AP is an adviser for Orphalan, Alexion, Vivet, and Univar and received institutional grants from Orphalan, Alexion, and AddMedica; ND-O is an adviser for Orphalan and Alexion. DD is an adviser for Orphalan and Alexion. CD is a consultant for TEVA Santé and received grants from TEVA Santé, Merz Pharma, ISIS Parkinson and ASDIA. MAO is an adviser for Orphalan. DR, CC, and JP have no conflicts of interest to declare. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- Poujois A., Mikol J., Woimant F. Wilson disease: brain pathology. Handb Clin Neurol. 2017;142:77–89. - PubMed
-
- Poujois A., Woimant F. Wilson’s disease: a 2017 update. Clin Res Hepatol Gastroenterol. 2018;42:512–520. - PubMed
-
- Walshe J.M. Cause of death in Wilson disease. Mov Disord. 2007;22:2216–2220. - PubMed
LinkOut - more resources
Full Text Sources

