Biallelic CRELD1 variants cause severe muscle weakness and infantile epilepsy
- PMID: 40980404
- PMCID: PMC12448699
- DOI: 10.1093/braincomms/fcaf326
Biallelic CRELD1 variants cause severe muscle weakness and infantile epilepsy
Abstract
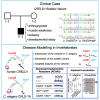
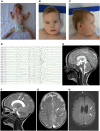
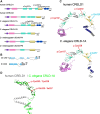
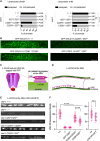
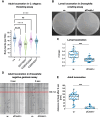
Nicotinic acetylcholine receptors are widely expressed in the peripheral and central nervous systems. Mutations in acetylcholine receptor-subunit genes have been associated with neuromuscular diseases, such as arthrogryposis multiplex congenita (AMC) and epilepsy. We report a patient with arthrogryposis, severe muscle weakness and neurodevelopmental delay. During his first year of life, he developed therapy-refractory epilepsy. Using whole-exome sequencing, we identified the compound pathogenic variants c. 875G>A (p. Cys292Tyr) and c. 959delA (p. Gln320Argfs*25) in the cysteine-rich with epidermal growth factor-like domain protein 1 gene (CRELD1, NM_001077415.3). Recently, functional studies have shown that CRELD1 is a membrane-associated endoplasmic reticulum-resident protein disulphide isomerase that acts as a maturation enhancer of AChR biogenesis, thereby controlling the abundance of functional receptors at the cell surface. To test pathogenicity, we took advantage of the genetics and extremely rapid genome editing in Caenorhabditis elegans. We were able to model these heterozygous variants and observed a decrease in AChRs at the neuromuscular junction. Hence, our study identifies compound heterozygous CRELD1 variants responsible for a rare neurodevelopmental disorder characterized by arthrogryposis, muscle weakness and epilepsy.
Keywords: CRELD1; arthrogryposis; epilepsy; nicotinic acetylcholine receptor.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures

References
-
- Colombo SF, Mazzo F, Pistillo F, Gotti C. Biogenesis, trafficking and up-regulation of nicotinic ACh receptors. Biochem Pharmacol. 2013;86(8):1063–1073. - PubMed
-
- Hurst R, Rollema H, Bertrand D. Nicotinic acetylcholine receptors: From basic science to therapeutics. Pharmacol Ther. 2013;137(1):22–54. - PubMed
-
- Gotti C, Clementi F. Neuronal nicotinic receptors: From structure to pathology. Prog Neurobiol. 2004;74(6):363–396. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
