Thalamocortical network neuromodulation for epilepsy
- PMID: 40980408
- PMCID: PMC12448812
- DOI: 10.1093/braincomms/fcaf270
Thalamocortical network neuromodulation for epilepsy
Abstract
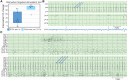
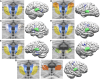
Despite the growing interest in network-guided neuromodulation for epilepsy, uncertainty about the safety and long-term efficacy of thalamocortical stimulation persists. Our evaluation focused on the use of a four-lead open-loop implantable pulse generator for thalamocortical network neuromodulation. We retrospectively reviewed seven patients with diverse seizure networks-poorly localized, regional, or multifocal-undergoing thalamocortical neuromodulation. Employing a four-lead system, electrodes targeted both thalamic and cortical seizure network nodes. We assessed seizure severity, life satisfaction and sleep quality on a 10-point scale, and seizure frequency was assessed via telephone interviews and chart review. Outcomes were assessed by the Wilcoxon sign-rank test at the 0.05 significance level. Seven patients with a median age at implant of 22 years (range 14-42 years) had a median disabling seizure reduction of 93% (range 50-100%, P = 0.0156), with 100% responder rate (≥50% reduction in seizure frequency) after a median of 17 months post-implantation (range 13-60). The median improvement in seizure severity was 3.5 out of 10 points (P = 0.0312), life satisfaction 4.5 points (P = 0.0312) and quality of sleep 3 points (P = 0.062). No perioperative complications occurred. Transient seizure exacerbations (n = 2) and stimulation-related sensory/motor side-effects (n = 2) quickly resolved with parameter adjustments. One patient required surgical revision due to delayed infection. Six patients had permanent electrode placement refined by intracranial EEG trial stimulation; five patients had >90% reduction in seizure frequency during trial stimulation. Thalamocortical network neuromodulation using a four-lead open-loop system is safe and associated with significant improvements in seizure control and patient quality of life. Trial stimulation during intracranial EEG shows promise for enhancing seizure network engagement and parameter optimization but requires further study. Prospective controlled trials are needed to further characterize and validate the efficacy and side-effect profile of thalamocortical network neuromodulation for epilepsy.
Keywords: cortical stimulation; deep brain stimulation.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
G.A.W., B.N.L., J.J.V.V., M.S. and B.H.B. declare intellectual property licensed to Cadence Neuroscience (B.N.L. waived contractual rights). B.N.L. declares intellectual property licensed to Seer Medical (contractual rights waived). G.A.W. licensed intellectual property and serves on the scientific advisor board of NeuroOne, Inc. B.N.L., G.A.W. and N.M.G. are investigators for the Medtronic Deep Brain Stimulation Therapy for Epilepsy Post-Approval Study. S.A. is a consultant for Blackrock Neurotech. B.N.L. is an investigator for the Neuroelectrics tDCS for Patients with Epilepsy Study. J.J.V.V., G.A.W., B.N.L. and N.M.G. are investigators for the NeuroPace RNS NAUTILUS study. K.L. is Chief Executive Officer and co-founder and D.S. is Chief Scientific Officer and co-founder of Cadence Neuroscience. N.M.G. has consulted for NeuroOne, Inc. (funds to Mayo Clinic). B.N.L. has consulted for Epiminder, Medtronic, Neuropace and Philips Neuro (all funds to Mayo Clinic). The remaining authors declare no competing interests.
Figures


References
-
- Kalilani L, Sun X, Pelgrims B, Noack-Rink M, Villanueva V. The epidemiology of drug-resistant epilepsy: A systematic review and meta-analysis. Epilepsia. 2018;59(12):2179–2193. - PubMed
-
- Kwan P, Arzimanoglou A, Berg AT, et al. Definition of drug resistant epilepsy: Consensus proposal by the ad hoc task force of the ILAE commission on therapeutic strategies. Epilepsia. 2010;51(6):1069–1077. - PubMed
-
- Morrell MJ; RNS System in Epilepsy Study Group . Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–1304. - PubMed
-
- Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51(5):899–908. - PubMed
-
- Fisher RS. Deep brain stimulation for epilepsy. Handb Clin Neurol. 2013;116:217–234. - PubMed
LinkOut - more resources
Full Text Sources
