Defining Relationships Among Tests for Kidney Transplant Antibody-Mediated Rejection
- PMID: 40980666
- PMCID: PMC12446986
- DOI: 10.1016/j.ekir.2025.06.017
Defining Relationships Among Tests for Kidney Transplant Antibody-Mediated Rejection
Abstract
Introduction: Effective therapies for kidney transplant antibody-mediated rejection (ABMR) will require accurate diagnoses plus assessment of ABMR activity, and the new tests that were used to show treatment effects in the clinical trial such as donor-derived cell-free DNA (dd-cfDNA) and molecular biopsy analysis (the Molecular Microscope Diagnostic System) could be useful.
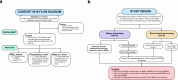
Methods: Trifecta-Kidney (ClinicalTrials.gov #NCT04239703) studied 717 indication biopsies to define the relationships among the following 4 tests used for ABMR: (i) standard-of-care (SOC) local histologic biopsy ABMR diagnosis, (ii) MMDx ABMR diagnosis, (iii) dd-cfDNA, and (iv) donor-specific antibody (DSA).
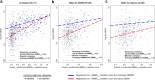
Results: All 4 tests were correlated in a partial correlation network, with a hierarchy of intertest correlations: MMDx ABMR > dd-cfDNA > histology ABMR > DSA. Surprisingly, DSA correlated at least as strongly with MMDx ABMR as with histologic ABMR, even though DSA is not used in MMDx. When expressed in the same 6 rejection classes, MMDx diagnosed ABMR more frequently than histology. When histology disagreed with MMDx ABMR, dd-cfDNA and DSA correlated more strongly with MMDx assessment. However, histology also detected ABMR lesions in some cases that MMDx called No Rejection, correlating with subthreshold molecular ABMR activity, dd-cfDNA and DSA (AJT 25:72-87, 2024). Molecular rejection predicted graft outcomes better than histologic rejection in Trifecta-Kidney, and this finding was confirmed in the earlier INTERCOMEX study cohort.
Discussion: The 4-way intertest correlations extend below current thresholds for diagnosing ABMR. These results map a network of 4 ABMR-related tests that can add precision to ABMR assessment in trials and clinical management, and highlight the need to establish the clinical significance of subthreshold ABMR activity.
Keywords: DSA; donor-derived cell-free DNA; kidney transplant; molecular diagnostics; rejection.
© 2025 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
-
- Haas M., Loupy A., Lefaucheur C., et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 2018;18:293–307. doi: 10.1111/ajt.14625. - DOI - PMC - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

