Mast Cell Association with the Microenvironment of a Phosphaturic Mesenchymal Tumour Secreting Fibroblast Growth Factor 23
- PMID: 40981193
- PMCID: PMC12452509
- DOI: 10.3390/medsci13030195
Mast Cell Association with the Microenvironment of a Phosphaturic Mesenchymal Tumour Secreting Fibroblast Growth Factor 23
Abstract
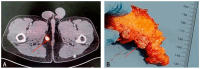
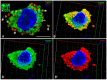
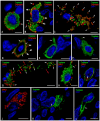
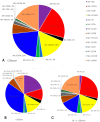
Background: Phosphaturic mesenchymal tumours secreting fibroblast growth factor 23 (hereinafter referred to as FGF23+ PMT) are rare neoplasms that can cause hypophosphataemic osteomalacia, owing to excessive FGF23 production. Mast cells (MCs) play a key role in tumour biology by modulating proliferative activity of atypical cells, resistance to innate and acquired immunity, angiogenesis, and metastatic behaviour. However, MCs associated with FGF23+ PMT have not previously been investigated. This study, to our knowledge, is the first to characterise features of the tumour microenvironment through spatial phenotyping of the immune and stromal landscape, together with histotopographic mapping of intercellular MC interactions with other subcellular populations in FGF23+ PMT. Methods: Histochemical staining (haematoxylin and eosin, toluidine blue, Giemsa solution, picro-Mallory protocol, silver impregnation), as well as monoplex and multiplex immunohistochemical staining with spatial phenotyping, were performed to detect atypical FGF23-secreting cells, immune cells (CD3, CD4, CD8, CD14, CD20, CD38, CD68, or CD163), stromal components (CD31, α-SMA, or vimentin), and specific MC proteases (tryptase, chymase, or carboxypeptidase A3). Bioinformatics analysis using artificial intelligence technologies was applied for spatial profiling of MC interactions with tumour, immunocompetent, and stromal cells in the tumour microenvironment. Results: Bioinformatic analysis of the entire tumour histological section, comprising over 70,000 cells stained using monoplex and multiplex immunohistochemical protocols, enabled identification of more than half of the cell population. The most abundant were CD14+ (30.7%), CD163+ (23.2%), and CD31+ (17.9%) cells. Tumour-associated MCs accounted for 0.7% of the total pool of immunopositive cells and included both mucosal and connective tissue subpopulations, predominantly of the tryptase + chymase-CPA3-specific protease phenotype. This pattern reflected combined multidirectional morphogenetic processes in the patient's FGF23+ PMT. More than 50% of MCs were colocalized with neighbouring cells of the tumour microenvironment within 20 μm, most frequently with monocytes (CD14+CD68+), M2 macrophages (CD68+CD163+), and endothelial cells (CD31+). In contrast, colocalization with atypical FGF23-secreting cells was rare, indicating minimal direct effects on tumour cell activity. Interaction with T lymphocytes, including CD8+, was also infrequent, excluding their activation and the development of antitumour effects. Mapping of MC histotopography validated the hypothesis of their inductive role in monocyte differentiation into M2 macrophages and probable polarisation of macrophages from M1 into M2, thereby contributing to slow tumour growth. MCs were further involved in extracellular matrix remodelling and participated in the formation of pro-osteogenic niches within the FGF23+ PMT microenvironment, leading to pathological osteoid development. Conclusions: This study demonstrated active MC participation in the evolution of the FGF23+ PMT microenvironment. The findings may be applied in translational medicine to develop novel algorithms for personalised therapy in patients with FGF23-secreting tumours, offering an alternative when surgical removal of the tumour is not feasible.
Keywords: carboxypeptidase A3; chymase; extracellular matrix; immune landscape; intercellular interaction; mast cells; multiplex immunohistochemistry; phosphaturic mesenchymal FGF23-secreting tumour; spatial phenotyping; tryptase; tumour microenvironment.
Conflict of interest statement
The authors declare that there are no conflicts of interest that could be perceived as prejudicing the impartiality of this article.
Figures






References
-
- Folpe A.L., Fanburg-Smith J.C., Billings S.D., Bisceglia M., Bertoni F., Cho J.Y., Econs M.J., Inwards C.Y., Jan de Beur S.M., Mentzel T., et al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: An analysis of 32 cases and a comprehensive review of the literature. Am. J. Surg. Pathol. 2004;28:1–30. doi: 10.1097/00000478-200401000-00001. - DOI - PubMed
-
- Kocelak P., Olszanecka-Glinianowicz M., Chudek J. Fibroblast growth factor 23--structure, function and role in kidney diseases. Adv. Clin. Exp. Med. 2012;21:391–401. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

