Mechanisms of Resistance to CAR T-Cells and How to Overcome Them
- PMID: 40981226
- PMCID: PMC12452515
- DOI: 10.3390/mps8050108
Mechanisms of Resistance to CAR T-Cells and How to Overcome Them
Abstract
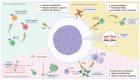
In the last few decades, chimeric antigen receptor (CAR) T-cell therapy has led to a paradigm shift in the treatment of hematological malignancies, including various subtypes of B-cell non-Hodgkin's lymphoma, B-cell acute lymphoblastic leukemia, and multiple myeloma. However, most patients experience refractoriness to CAR T-cells or relapse after treatment. Many efforts are underway to understand the mechanisms behind CAR T-cell failure, which are mainly related to CAR T-cell dysfunction, tumor-intrinsic resistance, an immunosuppressive tumor microenvironment, manufacturing issues, or patient-related factors. Several strategies are being developed to overcome these resistance mechanisms, including the engineering of more functional allogeneic CAR T-cell products, the targeting of alternative tumor antigens, and combination therapies with other drugs such as checkpoint inhibitors or small molecules to enhance CAR T-cell efficacy. In this review, we will provide an updated overview of the mechanisms of CAR T-cell failure and the therapeutic advances currently under development to address them.
Keywords: B-cell acute lymphoblastic leukemia; B-cell lymphoma; CAR NK; CAR T-cell resistance; CAR T-cells; allogeneic CAR T-cells; dual targeting; multiple myeloma.
Conflict of interest statement
MN—Abbvie, BMS, Istituto Gentili (advisory board); BB—Takeda, BMS, Incyte (advisory board); AB—Kite-Gilead, Novartis (advisory board); MC—Novartis, Incyte, Amgen, Servier, Otsuka, Italfarmaco, Abbvie, Astellas, Jazz (honoraria); RF—Kite-Gilead, Novartis, SOBI, Incyte, Otsuka (consultant and honoraria for talks); the other authors declare no conflicts of interest.
Figures
References
-
- Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. - DOI - PMC - PubMed
-
- Berdeja J.G., Madduri D., Usmani S.Z., Jakubowiak A., Agha M., Cohen A.D., Stewart A.K., Hari P., Htut M., Lesokhin A., et al. Ciltacabtagene Autoleucel, a B-Cell Maturation Antigen-Directed Chimeric Antigen Receptor T-Cell Therapy in Patients with Relapsed or Refractory Multiple Myeloma (CARTITUDE-1): A Phase 1b/2 Open-Label Study. Lancet. 2021;398:314–324. doi: 10.1016/S0140-6736(21)00933-8. - DOI - PubMed
-
- Neelapu S.S., Locke F.L., Bartlett N.L., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

