Conformational Effects in Intramolecular C(sp3)-H Bond Functionalization: Gold(I)-Catalyzed Cycloisomerization of Aliphatic 1-Bromoalkynes as Benchmark Reaction
- PMID: 40981713
- PMCID: PMC12501934
- DOI: 10.1021/acs.orglett.5c03430
Conformational Effects in Intramolecular C(sp3)-H Bond Functionalization: Gold(I)-Catalyzed Cycloisomerization of Aliphatic 1-Bromoalkynes as Benchmark Reaction
Abstract
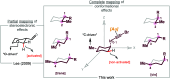
The use of cyclohexane derivatives displaying all possible substitution patterns has allowed us to study the influence of the conformational effects in the recently reported gold(I)-catalyzed cycloisomerization reaction of aliphatic 1-bromoalkynes that entails an unusual functionalization of a nonactivated C(sp3)-H bond. Some clear trends in terms of reactivity, regio- and stereoselectivity may be drawn from the experimental results and, in most cases, rationalized using intuitive conformational analysis arguments (i.e., equatorial/axial preferences). Occasionally, some unexpected results were also obtained, but they have also found a rational explanation. DFT calculations have been used to help us understand the underlying effects that dictate the reactivity or selectivity differences.
Figures
References
-
-
For selected examples of conformational effects on reactivity, see:
- Oshima T., Asahara H., Kubo E., Miyamoto S., Togaya K.. Conformational effects in acid-mediated ring opening of epoxides: A prominent role of the oxirane Walsh orbital. Org. Lett. 2008;10:2413–2416. doi: 10.1021/ol800535c. - DOI - PubMed
- Carnell A. J, Clegg W., Johnstone R. A.W, Parsy C. C, Sanderson W. R. 2-Bromocyclohexanone perhydrateX-ray crystal structure and conformational effects on reactivity in sulfoxidations. Tetrahedron. 2000;56:6571–6575. doi: 10.1016/S0040-4020(00)00608-6. - DOI
- Elbaum D., Porco J. A. Jr, Stout T. J., Clardy J., Schreiber S. L.. Stereochemical and conformational effects on the cycloaromatization of dynemicin A-related molecules. J. Am. Chem. Soc. 1995;117:211–225. doi: 10.1021/ja00106a025. - DOI
- Horton D., Priebe W., Sznaidman M. L.. Steric and conformational effects in the dehalogenation of 2-halo sugar derivatives with tributylstannane. J. Org. Chem. 1993;58:1821–1826. doi: 10.1021/jo00059a037. - DOI
- Jackson D. A., Smith D. C. C., Watt C. I. F.. Enhanced carbonyl reactivity and conformational effects in peri-dioxocycloalka[de]naphthalenes. J. Chem. Soc., Perkin Trans. 1. 1987;0:2437–2441. doi: 10.1039/p19870002437. - DOI
-
-
-
For selected examples of conformational effects on chemo- or regioselectivity, see:
- Wee A. G. H., Duncan S. C.. The rhodium(II)-catalyzed reaction of N-bis(trimethylsilylmethyl)diazoamides: Steric, electronic and conformational effects. Tetrahedron Lett. 2002;43:6173–6176. doi: 10.1016/S0040-4039(02)01273-X. - DOI
- Kim D., Lee J., Shim P. J., Lim J. I., Doi T., Kim S.. Role of conformational effects on the regioselectivity of macrocyclic INOC reactions: Two new asymmetric total syntheses of (+)-brefeldin A. J. Org. Chem. 2002;67:772–781. doi: 10.1021/jo010744a. - DOI - PubMed
- Doyle M. P., Kalinin A. V.. Highly enantioselective route to β-lactams via intramolecular C–H insertion reactions of diazoacetylazacycloalkanes catalyzed by chiral dirhodium(II) carboxamidates. Synlett. 1995;1995:1075–1076. doi: 10.1055/s-1995-5176. - DOI
-
-
-
For selected examples of conformational effects on diastereoselectivity, see:
- Janody S., Jazzar R., Comte A., Holstein P. M., Vors J.-P., Ford M. J., Baudoin O.. Synthesis of 1-indanols and 1-indanamines by intramolecular palladium(0)-catalyzed C(sp3)–H arylation: Impact of conformational effects. Chem.Eur. J. 2014;20:11084–11090. doi: 10.1002/chem.201402907. - DOI - PubMed
- Marshall J. A., Beaudoin S.. Remote conformational bias effects on diastereofacial selectivity in SE2′ additions of γ-oxygenated allylic stannanes to chiral enals. J. Org. Chem. 1994;59:7833–7838. doi: 10.1021/jo00104a047. - DOI
-
-
-
For reviews on conformational effects on selectivity, see:
- Ring A., Ford A., Maguire A. R.. Substrate and catalyst effects in C–H insertion reactions of α-diazoacetamides. Tetrahedron Lett. 2016;57:5399–5406. doi: 10.1016/j.tetlet.2016.10.081. - DOI
- Merlic C. A., Zechman A. L.. Selectivity in rhodium(II)-catalyzed reactions of diazo compounds: Effects of catalyst electrophilicity, diazo substitution, and substrate substitution. From chemoselectivity to enantioselectivity. Synthesis. 2003;2003:1137–1156. doi: 10.1055/s-2003-39389. - DOI
-
-
-
For selected studies on conformational effects in CH functionalization reactions, see:
- Yun S. Y., Zheng J.-C., Lee D.. Stereoelectronic effect for the selectivity in C–H insertion of alkylidene carbenes and its application to the synthesis of platensimycin. J. Am. Chem. Soc. 2009;131:8413–8415. doi: 10.1021/ja903526g. - DOI - PubMed
- Zheng J.-C., Yun S.-W., Sun C., Lee N. K., Lee D.. Selectivity control in alkylidene carbene-mediated C–H insertion and allene formation. J. Org. Chem. 2011;76:1086–1099. doi: 10.1021/jo102180f. - DOI - PubMed
- Flanigan D. L., Yoon C. H., Jung K. W.. Synthesis of chiral γ-lactams via Rh(II) catalyzed intramolecular C–H insertion: α-Substituents and conformational effects. Tetrahedron Lett. 2005;46:143–146. doi: 10.1016/j.tetlet.2004.10.159. - DOI
- Yoon C. H., Zaworotko M. J., Moulton B., Jung K. W.. Regio- and stereocontrol elements in Rh(II)-catalyzed intramolecular C–H insertion of α-diazo-α-(phenylsulfonyl)acetamides. Org. Lett. 2001;3:3539–3542. doi: 10.1021/ol016647l. - DOI - PubMed
- Wee A. G. H., Liu B., Zhang L.. Dirhodium tetraacetate catalyzed carbon-hydrogen insertion reaction in N-substituted α-carbomethoxy-α-diazoacetanilides and structural analogs. Substituent and conformational effects. J. Org. Chem. 1992;57:4404–4414. doi: 10.1021/jo00042a018. - DOI
-
LinkOut - more resources
Full Text Sources