Factors in the Initial Resuscitation of Patients With Severe Trauma: The FiiRST-2 Randomized Clinical Trial
- PMID: 40982282
- PMCID: PMC12455389
- DOI: 10.1001/jamanetworkopen.2025.32702
Factors in the Initial Resuscitation of Patients With Severe Trauma: The FiiRST-2 Randomized Clinical Trial
Erratum in
Abstract
Importance: Patients with bleeding and coagulopathic trauma often require more transfusions and have higher mortality rates, motivating research on improving hemostatic strategies.
Objective: To evaluate the replacement of clotting factors with frozen plasma (FP) or factor concentrates (fibrinogen concentrate [FC] and prothrombin complex concentrate [PCC]) in the initial resuscitation of patients with trauma.
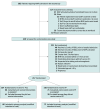
Design, setting, and participants: This multicenter, parallel-control, superiority randomized clinical trial was conducted at 6 level I trauma centers in Canada, between April 2021 and February 2023. Eligible patients were those with massive hemorrhage protocol (MHP) activation on admission and aged 16 years or older. Patients were excluded if they received more than 2 red blood cell (RBC) units either before hospital admission or in the hospital prior to randomization or if they had a catastrophic head injury. Follow-up was completed on March 25, 2023. The primary analysis was based on the modified intention-to-treat approach.
Interventions: The intervention group received FC 4 g and PCC 2000 IU in MHP packs 1 and 2. The control group received 4 FP units. Concurrently, patients received 4 RBC units (both packs) and 1 dose of platelets (pack 2). After pack 2, FP was administered at clinician discretion.
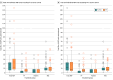
Main outcomes and measures: Primary outcome was the number of allogeneic blood product (RBC, FP, and platelet) units administered within 24 hours. Secondary outcomes included incidence of thromboembolic events, duration of intensive care unit stay, and mortality.
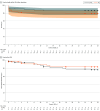
Results: Of the 217 patients enrolled, 107 were randomly assigned to the FC-PCC group and 110 to the FP group; 137 patients were included in the primary analysis (66 in the FC-PCC group and 71 in the FP group). Baseline characteristics were similar between groups (median [IQR] age, 38 [29-55] years; 111 males [81.0%]). Among these patients, 95 (69.3%) had blunt mechanisms of injury, and the median (IQR) Injury Severity Score was 29 (19-43). Mean 24-hour transfusions were 20.8 (95% CI, 16.7-25.9) units in the FC-PCC group and 23.8 (95% CI, 19.2-29.4) units in the FP group. The mean ratio was 0.87 (1-sided 97.5% CI, 0.00-1.19; P = .20 for superiority). No significant differences were found in thromboembolic complications or 24-hour and 28-day mortality. The trial was terminated after the interim analysis showed conditional power of less than 25%, requiring an impractically large sample to show superiority.
Conclusions and relevance: In this randomized clinical trial, clotting factor concentrates were not superior to FP for initial resuscitation of patients with trauma. Efficacy and safety outcomes were similar across the treatment groups.
Trial registration: ClinicalTrials.gov Identifier: NCT04534751.
Conflict of interest statement
Figures
Comment in
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

