Long-Term Personalized Adaptive Deep Brain Stimulation in Parkinson Disease: A Nonrandomized Clinical Trial
- PMID: 40982287
- PMCID: PMC12455485
- DOI: 10.1001/jamaneurol.2025.2781
Long-Term Personalized Adaptive Deep Brain Stimulation in Parkinson Disease: A Nonrandomized Clinical Trial
Abstract
Importance: Adaptive deep brain stimulation (aDBS) automatically adjusts stimulation amplitude in response to changes in relevant neural activity in people with Parkinson disease (PD). Whether long-term at-home aDBS is safe and delivers effective therapy in people with PD remains unknown.
Objective: To determine the tolerability, efficacy, and safety of long-term aDBS in people with PD who were previously stable receiving continuous DBS (cDBS).
Design, setting, and participants: This international, open-label, prospective, pivotal trial enrolled participants from December 2020 to July 2022 in the US, Canada, and Europe. Referred participants with PD were first assessed while receiving stable cDBS and those who tolerated 2 aDBS modes were randomized and blinded to 30 days in each mode (single-blind crossover design); those who tolerated only 1 mode were assessed in that mode only; assessments completed holding medication stable. Participants were given the option to continue their selected mode of aDBS for long-term follow-up (10 months). Data used for analysis were from March 2024. Multiple imputation was used if more than 5% of data was missing for the primary or secondary end points. A referred sample of 68 participants with PD, stable while receiving cDBS and medication, was included.
Interventions: Two modes of aDBS controlled by an embedded closed-loop stimulation system: single threshold (ST-aDBS) and dual threshold (DT-aDBS).
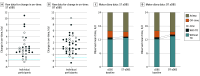
Main outcomes and measures: The primary end point required that at least 50% of participants meet a performance goal of on-time (ie, time when symptoms were well controlled) without troublesome dyskinesias with no less than 1-SD reduction (and post hoc threshold less than 2 hours per day reduction) reported during aDBS therapy compared to cDBS, determined from a self-reported motor diary. The secondary end point was total electrical energy delivered (TEED) compared between aDBS and cDBS. Safety assessments were conducted by characterizing adverse events (AEs), stimulation-related AEs, serious AEs, and device deficiencies.
Results: A total of 68 participants enrolled (mean [SD] age, 62.2 [8.4] years; 48 [70.6%] male); 40 and 35 were evaluated with DT-aDBS and ST-aDBS, respectively. The primary end point performance goal was met in the DT-aDBS group (91% of participants) and ST-aDBS (79% of participants) with the post hoc performance threshold; no difference between aDBS modes (χ21 = 1.0; P = .51). TEED was reduced during ST-aDBS compared to cDBS (mean change, -15%; nominal P = .01) and not different from DT-aDBS. All but 1 stimulation-related AE resolved during the aDBS setup and adjustment phase with no serious device AEs through long-term follow-up. Exploratory analyses suggested improvement in on-time without troublesome dyskinesias with DT-aDBS compared to cDBS.
Conclusions and relevance: In this study, long-term aDBS was tolerable, effective, and safe in people with PD who were previously stable while receiving cDBS.
Trial registration: ClinicalTrials.gov Identifier: NCT04547712.
Conflict of interest statement
Figures
References
-
- Fox SH, Katzenschlager R, Lim SY, et al. ; Movement Disorder Society Evidence-Based Medicine Committee . International Parkinson and movement disorder society evidence-based medicine review: Update on treatments for the motor symptoms of Parkinson’s disease. Mov Disord. 2018;33(8):1248-1266. doi: 10.1002/mds.27372 - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

