Ninjurin-1 mediates cell lysis and detrimental inflammation of PANoptosis during influenza A virus infection
- PMID: 40983621
- PMCID: PMC12454662
- DOI: 10.1038/s41392-025-02391-9
Ninjurin-1 mediates cell lysis and detrimental inflammation of PANoptosis during influenza A virus infection
Abstract
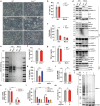
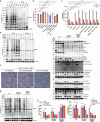
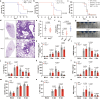
Influenza A virus (IAV) induces ZBP1-mediated PANoptosis, a form of lytic inflammatory cell death characterized by concurrent activation of the pyroptosis, necroptosis and apoptosis pathways. Ninjurin-1 (NINJ1) is a recently identified mediator of plasma membrane rupture but functions diversely in different types of cell death. However, little is known about the role of NINJ1 in IAV-induced PANoptosis and viral pneumonia. Here, we report that IAV infection triggered an increase in the expression of NINJ1, which then oligomerized and mediated cell lysis in infected macrophages. The deficiency of NINJ1 prevented plasma membrane rupture and the release of DAMPs and IL-1β without affecting the progression of cell death. Activation of any single PANoptosis pathway was sufficient to trigger the oligomerization of NINJ1 and robust cell lysis. Accordingly, only when all PANoptosis pathways were concurrently blocked could the oligomerization of NINJ1, cell death, and cell rupture be prevented. Ablation of NINJ1 in vivo also alleviated IAV-induced lung injury and mortality. Furthermore, we revealed an association between NINJ1 upregulation and poor outcomes in patients with COVID-19. Collectively, our findings indicate a pivotal role of NINJ1 in the immunopathology of IAV infection and its potential as a bioindicator of disease severity and prognosis in viral pneumonia and viral sepsis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

