MT1-MMP inhibition rejuvenates ageing brain and rescues cognitive deficits in obesity
- PMID: 40983624
- PMCID: PMC12454644
- DOI: 10.1038/s41421-025-00825-w
MT1-MMP inhibition rejuvenates ageing brain and rescues cognitive deficits in obesity
Abstract
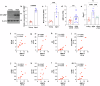
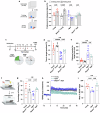
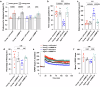
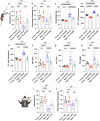
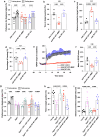
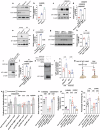
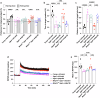
Obesity has been linked to an increased risk of cognitive impairment and dementia in later life. Although aging and obesity are both associated with cognitive decline, it remains unclear how they interact to affect cognitive function across the lifespan and how brain function might mediate their relationship with cognition. Our previous findings and other studies have shown that membrane type 1-matrix metalloproteinase (MT1-MMP/MMP14), which increases with age, regulates energy homeostasis. Inhibiting MT1-MMP improves insulin sensitivity, reduces body fat, and lowers serum cholesterol. Here, we demonstrate that MT1-MMP links neuroinflammation to cognitive decline in aging and obesity. Inflammatory responses in the brain increase MT1-MMP activation in the hippocampus of both mice and humans. Activation of hippocampal MT1-MMP alone can trigger cognitive decline and synaptic impairment independently of neuroinflammation. Conversely, ablation of MT1-MMP in the hippocampus reverses cognitive decline and improves synaptic plasticity in aging and obesity. Pharmacological inhibition of MT1-MMP, through an orally administered brain-penetrant inhibitor or targeted delivery of a neutralizing antibody to the hippocampus, improves memory and learning in aged and obese mice without toxicity. Mechanistically, MT1-MMP proteolytically inactivates G-protein-coupled receptor 158 (GPR158), a hippocampal receptor for osteocalcin (OCN) that is important for the maintenance of cognitive integrity, thus suppressing the ability of the OCN-GPR158 axis to promote cognition in aging and obesity. These findings suggest a new mechanism underlying hippocampal dysfunction and reveal the potential for treating multiple age-related diseases, including neurodegenerative disorders, obesity, diabetes, and atherosclerosis, with a single MT1-MMP-blocking agent.
© 2025. The Author(s).
Conflict of interest statement
Conflict of interest: The authors declare no competing interests.
Figures
References
-
- Hedden, T. & Gabrieli, J. D. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci.5, 87–96 (2004). - PubMed
Grants and funding
- 12102020/Research Grants Council, University Grants Committee (RGC, UGC)
- 08793626/Food and Health Bureau of the Government of the Hong Kong Special Administrative Region | Health and Medical Research Fund (HMRF)
- ITS/058/22MS/Innovation and Technology Commission (ITF)
- 32322091/National Science Foundation of China | National Natural Science Foundation of China-Yunnan Joint Fund (NSFC-Yunnan Joint Fund)
- 2025A03J4482/Guangzhou Science, Technology and Innovation Commission (Bureau of Science and Information Technology of Guangzhou Municipality)
LinkOut - more resources
Full Text Sources

