Macrophage ferroptosis potentiates GCN2 deficiency induced pulmonary venous arterialization
- PMID: 40983649
- PMCID: PMC12454641
- DOI: 10.1038/s41467-025-64035-4
Macrophage ferroptosis potentiates GCN2 deficiency induced pulmonary venous arterialization
Abstract
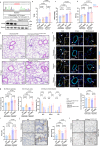
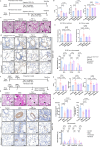
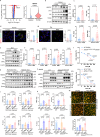
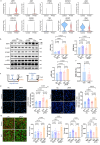
Pulmonary veno-occlusive disease (PVOD) is a fatal disease characterized by the remodelling of pulmonary veins and haemosiderin accumulation in macrophages. Although (General Control Nonderepressible 2) GCN2 deficiency has been reported in PVOD patients, the underlying mechanism by which GCN2 deficiency affects the pulmonary venous cells and the surrounding cells, remains unclear. Here, we perform immunohistochemistry and scRNA-sequencing analyses to show that macrophages are the major population affected by GCN2 deficiency and ferroptosis pathway-related genes are upregulated in lung macrophages of PVOD patients. Treatment with the specific ferroptosis inhibitor ferrostatin-1 (Fer-1) reverses the changes in haemodynamic indices observed in Eif2ak4K1488X/K1488X hypoxia mice and PVOD model rats. Furthermore, GCN2 deficiency increases HMOX1 and iron levels to facilitate ferroptosis in macrophages, and enhances arterial marker expression in venous endothelial cells (VECs). Specifically, spatial transcriptome analysis shows increased expression of NRP1, KDR and EFNB2 through ETS1 in VECs from PVOD patients. Our findings suggest the potential of targeting macrophage ferroptosis as a therapeutic strategy for treating related vascular diseases, and of using NRP1/KDR/EFNB2 expression as a specific marker set for venous arterialization.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Chaisson, N. F., Dodson, M. W. & Elliott, C. G. Pulmonary capillary hemangiomatosis and pulmonary veno-occlusive disease. Clin. Chest Med.37, 523–534 (2016). - PubMed
-
- Manaud, G. et al. Comparison of human and experimental pulmonary veno-occlusive disease. Am. J. Respir. Cell Mol. Biol.63, 118–131 (2020). - PubMed
-
- Montani, D. et al. Pulmonary veno-occlusive disease. Eur. Respir. J.47, 1518–1534 (2016). - PubMed
MeSH terms
Substances
Grants and funding
- 2023YFC2507103/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 2021YFA1100500/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 2023YFC2507100/Ministry of Science and Technology of the People's Republic of China (Chinese Ministry of Science and Technology)
- 82470431/National Natural Science Foundation of China (National Science Foundation of China)
- 81670054/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

