The relationship of soluble tau species with Alzheimer's disease amyloid plaque removal and tau pathology
- PMID: 40985290
- PMCID: PMC12455363
- DOI: 10.1002/alz.70689
The relationship of soluble tau species with Alzheimer's disease amyloid plaque removal and tau pathology
Abstract
Background: Tau-derived cerebrospinal fluid (CSF) biomarkers correlate with amyloid-beta (Aβ) plaques or tau tangles in Alzheimer's disease (AD). This study assessed the effects of long-term anti-Aβ antibodies on amyloid plaques, tau tangles, and CSF tau species to determine the relationships between them.
Methods: A post-hoc analysis of the DIAN-TU-001 trial (NCT01760005) examined 142 participants at risk for dominantly inherited AD randomized to solanezumab (n = 50), gantenerumab (n = 52), or placebo (n = 40). High-resolution mass spectrometry quantified CSF tau species over four years.
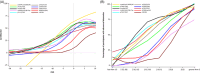
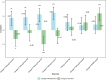
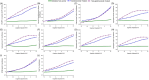
Results: Phosphorylated tau (p-tau) species (153, 181, 217, 231) increased early in preclinical AD but were reduced with gantenerumab-mediated Aβ plaque reduction. Nearly a decade later, MTBR-tau243 and p-tau205 increased, showing no association with Aβ reduction, aligning with tau tangle pathology progression.
Discussion: Initially changing soluble p-tau species track Aβ plaque reduction, while ptau205 and MTBR-243 reflect tau tangle pathology, informing different pathways of therapeutic strategies.
Highlights: p-tau217 and p-tau231 correlate with Aβ-PET and respond to Aβ-plaque lowering therapies. Aβ immunotherapy trials support a direct link between p-tau changes and Aβ plaques Gantenerumab reduces Aβ plaques but does not affect tau NFT-related biomarkers. Blood-based p-tau217 assays may provide a non-invasive tool to monitor Aβ therapies. MTBR-tau243 strongly correlates with tau PET and tracks NFT pathology progression. Further studies are needed to validate tau biomarkers for tracking NFT-targeting therapies.
Keywords: amyloid beta plaque reduction; dominantly inherited Alzheimer's disease; microtubule‐binding region; phosphorylated tau.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
There are several inventions that have been filed by Washington University for patents, including “Methods of diagnosing AD with phosphorylation changes” and “Methods to detect MTBR‐tau isoforms and use”. These intellectual properties owned by Washington University can be or are licensed and some licensing income may be distributed to Drs. Barthelemy, Bateman, McDade and other inventors. These intellectual properties being licensed by Washington University from C2N and currently being utilized in our research have been reviewed by the Washington University COI and ICOI committees.
All co‐inventors, including some lab members, the University, and Drs. Barthélemy, Bateman, McDade could receive part of the profits from any sales of these tests by C2N, which is in the process of licensing or has licensed some IP from the University. These activities have been reviewed by Washington University's (WU) Conflicts of Interest Review Committee in accordance with WU's Research Conflicts of Interest Policy and WU's Institutional Conflict of Interest Review Committee in accordance with WU's Institutional Conflict of Interest Policy.
Figures

References
-
- Salvadó G, Horie K, Barthélemy NR, et al. Novel CSF tau biomarkers can be used for disease staging of sporadic Alzheimer's disease. medRxiv [Internet]. 2023. http://medrxiv.org/content/early/2023/07/16/2023.07.14.23292650.abstract. 2023.07.14.23292650
Publication types
MeSH terms
Substances
Grants and funding
- U01AG042791/AG/NIA NIH HHS/United States
- U01AG042791-S1/AG/NIA NIH HHS/United States
- R01AG046179/AG/NIA NIH HHS/United States
- R01AG053267-S1/AG/NIA NIH HHS/United States
- U19AG032438/AG/NIA NIH HHS/United States
- K23AG046363/AG/NIA NIH HHS/United States
- P30AG066444(pilot35.3)/AG/NIA NIH HHS/United States
- DIAN-TTU-12-243040/ALZ/Alzheimer's Association/United States
- SG-20-690363-DIAN/ALZ/Alzheimer's Association/United States
- AARF-16-443265/ALZ/Alzheimer's Association/United States
- Eli Lilly and Company
- F. Hoffmann-La Roche Ltd.
- Avid Radiopharmaceuticals
- GHR Foundation
- Anonymous organization
- Cogstate (in-kind)
- Signant Health (in-kind)
- German Center for Neurodegenerative Diseases
- Raul Carrea Institute for Neurological Research (FLENI)
- Japan Agency for Medical Research and Development
- Korea Health Technology R&D Project, Korea Health Industry Development Institute (KHIDI)Korea Health Industry Development Institute (KHIDI)
- Spanish Institute of Health Carlos III
- CAPMC/ CIHR/Canada
- Canadian Consortium of Neurodegeneration and Aging
- Brain Canada Foundation
- Fonds de Recherche du Québec - Santé
- Eisai Incorporated
LinkOut - more resources
Full Text Sources
Medical

