Hierarchical encoding of natural sound mixtures in ferret auditory cortex
- PMID: 40985339
- PMCID: PMC12456947
- DOI: 10.7554/eLife.106628
Hierarchical encoding of natural sound mixtures in ferret auditory cortex
Abstract
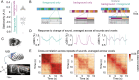
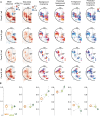
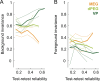
Extracting relevant auditory signals from complex natural scenes is a fundamental challenge for the auditory system. Sounds from multiple sources overlap in time and frequency. In particular, dynamic 'foreground' sounds are often masked by more stationary 'background' sounds. Human auditory cortex exhibits a hierarchical organization where background-invariant representations are progressively enhanced along the processing stream, from primary to non-primary regions. However, we do not know whether this organizational principle is conserved across species and which neural mechanisms drive this invariance. To address these questions, we investigated background invariance in ferret auditory cortex using functional ultrasound imaging, which enables large-scale, high-resolution recordings of hemodynamic responses. We measured responses across primary, secondary, and tertiary auditory cortical regions as ferrets passively listened to mixtures of natural sounds and their components in isolation. We found a hierarchical gradient of background invariance, mirroring findings in humans: responses in primary auditory cortex reflected contributions from both foreground and background sounds, while background invariance increased in higher-order auditory regions. Using a spectrotemporal filter-bank model, we found that in ferrets this hierarchical structure could be largely explained by tuning to low-order acoustic features. However, this model failed to fully account for background invariance in human non-primary auditory cortex, suggesting that additional, higher-order mechanisms are crucial for background segregation in humans.
Keywords: auditory cortex; background invariance; cortical hierarchy; cross-species; ferret; natural sounds; neuroscience.
© 2025, Landemard et al.
Conflict of interest statement
AL, CB, YB No competing interests declared
Figures









Update of
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

