Investigation of the Impact of Tryptophan-Metabolizing Enzymes and Kynurenic Acid on Antibody-Mediated Glomerulonephritis
- PMID: 40985497
- PMCID: PMC12455905
- DOI: 10.1096/fj.202501800R
Investigation of the Impact of Tryptophan-Metabolizing Enzymes and Kynurenic Acid on Antibody-Mediated Glomerulonephritis
Abstract
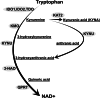
Tryptophan (TRP) metabolism through the kynurenine pathway generates multiple biologically active metabolites with diverse immunomodulatory effects, but their roles in glomerulonephritis (GN), particularly in innate immunity, remain poorly understood. Using a nephrotoxic serum-induced GN (NTS-GN) model, we first analyzed mice deficient in key TRP-metabolizing enzymes of the kynurenine pathway: Indoleamine 2,3-dioxygenase 1 and 2 (IDO1 and IDO2), and kynurenine 3-monooxygenase (KMO), and found that Ido1-deficient mice exhibited exacerbated kidney injury and glomerular neutrophil infiltration, whereas Ido2 deficiency had no significant impact. In contrast, Kmo-deficient mice showed reduced crescent formation. Unexpectedly, the concentration of kynurenic acid (KYNA), a downstream metabolite of IDO1, was elevated in the kidney cortex of Ido1-deficient mice. Exogenous KYNA administration improved survival, ameliorated renal injury, and reduced neutrophil infiltration in Ido1-deficient mice, indicating its protective effect against antibody-mediated injury. Moreover, KYNA suppressed immune complex-mediated neutrophil spreading, attenuated FcγR-dependent Syk phosphorylation, and reduced VEGF secretion in vitro. Our results position KYNA as a key modulator of neutrophil-driven inflammation in antibody-mediated GN. This study uncovers distinct roles for kynurenine pathway enzymes and highlights the TRP-KYNA pathway as a promising immunometabolic target for controlling innate immune responses in GN.
Keywords: Indoleamine 2,3‐dioxygenase; RRID:AB_2337118; RRID:IMSR_JAX:005867; RRID:MGI:2159965; RRID:MGI:3028467; RRID:MGI:5759308; glomerulonephritis; kynurenic acid; neutrophils; tryptophan.
© 2025 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Shimizu T., Nomiyama S., Hirata F., and Hayaishi O., “Indoleamine 2,3‐Dioxygenase. Purification and Some Properties,” Journal of Biological Chemistry 253, no. 13 (1978): 4700–4706. - PubMed
-
- Ball H. J., Sanchez‐Perez A., Weiser S., et al., “Characterization of an Indoleamine 2,3‐Dioxygenase‐Like Protein Found in Humans and Mice,” Gene 396, no. 1 (2007): 203–213. - PubMed
-
- Munn D. H., Zhou M., Attwood J. T., et al., “Prevention of Allogeneic Fetal Rejection by Tryptophan Catabolism,” Science 281, no. 5380 (1998): 1191–1193. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

