Preferences of Dutch Parents and Expectant Parents for Respiratory Syncytial Virus Prevention Strategies: A Discrete Choice Experiment
- PMID: 40986175
- PMCID: PMC12511510
- DOI: 10.1007/s40121-025-01214-2
Preferences of Dutch Parents and Expectant Parents for Respiratory Syncytial Virus Prevention Strategies: A Discrete Choice Experiment
Abstract
Introduction: Respiratory syncytial virus (RSV) is the leading cause of acute lower respiratory tract infection in infants. This study examined the preferences of Dutch parents and expectant parents for two RSV prevention strategies for infant protection: a maternal vaccine versus an infant monoclonal antibody (mAb) injection.
Methods: An online survey including a discrete choice experiment was conducted. Participants chose between two immunisation options for 'a common virus among infants' that represented RSV. These differed based on six attributes: timing and recipient of the injection, costs, recommended by a healthcare provider (HCP), included in the National Immunisation Programme (NIP), administration location, and co-administered with other injections. The main outcomes were preference weights, conditional relative attribute importance (CRAI), and willingness to be immunised.
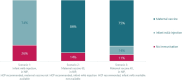
Results: The survey was completed by 150 participants (90% female; 49% parents; 51% expectant parents; mean age 31.23 ± 5.61 years). Participants preferred an immunisation option that is administered to pregnant women [mean = 1.48 (95% confidence interval (CI) 1.18-1.82)], free of charge [mean = 1.36 (95% CI 1.10-1.67)], recommended by an HCP [mean = 0.50 (95% CI, 0.34-0.66)], and included in the NIP [mean = 0.42 (95% CI, 0.26-0.58)]. The most important attributes were timing and recipient of the injection [CRAI = 32% (95% CI, 28-35%)] and costs [CRAI = 24% (95% CI, 20-28%)]. Willingness to be immunised was higher when the maternal vaccine and infant mAb injection were in the NIP than when only the infant mAb injection was available (89% vs 74%).
Conclusions: The results suggest that most Dutch parents and expectant parents would prefer a maternal vaccine to an infant mAb injection to immunise their infants against an RSV-like virus. An NIP that incorporates both strategies may enhance uptake and protect the most infants. However, as the attributes were not exhaustively or explicitly presented in the context of RSV prevention, the results may not be completely transferable.
Keywords: Discrete choice experiment; Infant immunisation; Monoclonal antibody; Nirsevimab; Preference; Pregnancy; RSVpreF maternal vaccination; Respiratory syncytial virus; Vaccine.
© 2025. Pfizer Inc and The Authors under exclusive licence to Springer Healthcare Ltd., part of Springer Nature.
Conflict of interest statement
Declarations. Conflict of Interest: Annefleur C. Langedijk, Floris van den Dungen, and Diana Mendes are employees of Pfizer and may hold Pfizer stocks. M. Claire Verhage and Elise Kocks are employees of SKIM, an independent market research company that received financial support from Pfizer to conduct this research. Marlies van Houten reported the following disclosures: investigator-initiated studies with Pfizer, Sanofi, Moderna, and GSK, participation with Pfizer (MATISSE Study), and consultancy with Pfizer, MSD, Sanofi, Moderna, and GSK. Lisette Harteveld, Lisanne van Leeuwen, Lucy Smit, and Jennie van den Boer declare that they have no competing interests. Ethical Approval: The study was conducted in accordance with the guidelines of the European Pharmaceutical Market Research Association and the Dutch Market Research Association. Ethical approval for this study was not required, in accordance with the Dutch Medical Research Involving Human Subjects Act. Participants provided informed consent to participate in the study (electronic checkbox consent).
Figures


References
-
- Heemskerk S, van Heuvel L, Asey T, Bangert M, Kramer R, Paget J, van Summeren J. Disease burden of RSV infections and bronchiolitis in young children (< 5 years) in primary care and emergency departments: A systematic literature review. Influenza Other Respir Viruses. 2024;18(8): e13344. 10.1111/irv.13344. - PMC - PubMed
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet. 2022;399(10340):2047–64. 10.1016/S0140-6736(22)00478-0. - PMC - PubMed
-
- National Health Service: Respiratory syncytial virus (RSV). https://www.nhs.uk/conditions/respiratory-syncytial-virus-rsv/ (2024). Accessed 29 Oct 2024.
-
- European Centre for Disease Prevention and Control: Respiratory syncytial virus (RSV). https://www.ecdc.europa.eu/en/respiratory-syncytial-virus-rsv (2024). Accessed 29 Oct 2024.
LinkOut - more resources
Full Text Sources
Miscellaneous

