Cost-Effectiveness Analysis of Aztreonam-Avibactam (ATM-AVI) Versus Colistin + Meropenem (COL + MER) for the Treatment of Infections Caused by Metallo-β-Lactamase (MBL)-Producing Enterobacterales in Italy
- PMID: 40986278
- PMCID: PMC12602586
- DOI: 10.1007/s40273-025-01528-6
Cost-Effectiveness Analysis of Aztreonam-Avibactam (ATM-AVI) Versus Colistin + Meropenem (COL + MER) for the Treatment of Infections Caused by Metallo-β-Lactamase (MBL)-Producing Enterobacterales in Italy
Abstract
Background and objective: Aztreonam-avibactam (ATM-AVI) is a novel combination antibiotic approved in Europe for the treatment of complicated intra-abdominal infection, hospital-acquired pneumonia, including ventilator-associated pneumonia; complicated urinary tract infection, including pyelonephritis and for infections due to aerobic Gram-negative organisms with limited treatment options. This analysis assessed the cost effectiveness of ATM-AVI ± metronidazole versus colistin + meropenem (COL + MER) for the treatment of patients with complicated intra-abdominal infection and hospital-acquired pneumonia/ventilator-associated pneumonia, including infections with suspected metallo-β-lactamase-producing Enterobacterales from the public payer perspective in Italy using phase III trial data.
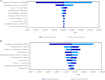
Methods: The cost-effectiveness analysis adopted a decision tree model to simulate the clinical pathway of complicated intra-abdominal infection and hospital-acquired pneumonia/ventilator-associated pneumonia, followed by a Markov model to capture lifetime health outcomes on cured patients, with costs valued in 2024 Euros and discounted at 3%. The model captures the impact of resistant pathogens and side effects (i.e. nephrotoxicity). Model uncertainty was assessed using a probabilistic and deterministic sensitivity analysis.
Results: The ATM-AVI treatment sequence (ATM-AVI ± metronidazole followed by cefiderocol after treatment failure) had improved clinical outcomes and higher cure rates, shorter hospital stays and higher quality-adjusted life-year gains compared with the COL + MER sequence (COL + MER followed by cefiderocol after treatment failure). The incremental cost-effectiveness ratio in the ATM-AVI sequence was dominant for complicated intra-abdominal infection and was €1552 per quality-adjusted life-year for hospital-acquired pneumonia/ventilator-associated pneumonia, well below the willingness-to-pay threshold of €30,000 in Italy.
Conclusions: Our analysis suggests that ATM-AVI is expected to be a cost-effective use of Italian healthcare resources for treating suspected metallo-β-lactamase-producing Enterobacterales, including complicated intra-abdominal infection and hospital-acquired pneumonia/ventilator-associated pneumonia.
Plain language summary
Bacterial resistance happens when antibiotics no longer kill bacteria as they should. This problem is getting worse, especially for serious infections that are hard to treat. Aztreonam-avibactam (called antibiotic A) is a new medicine used in hospitals to treat these infections, including those in the belly and lungs. This study looked at how well antibiotic A. It was compared to another treatment (colistin plus meropenem, called antibiotic B). A computer model was used to study the results and costs in Italy’s public healthcare system. It looked at how many people got better, how often the infection came back, side effects, how long people lived in good health and the cost of care for each of these antibiotics. The study found that people who got antibiotic A lived longer, had better health and cost less to treat than those who got antibiotic B. This was true in many different cases. This suggests that antibiotic A gives better results at a fair cost for treating serious hospital infections in Italy.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Funding: This work was funded by Pfizer. Work by Source Health Economics was funded by Pfizer. Editorial and medical writing assistance was provided by Kelly O’Toole and Phillipa White, of Source Health Economics. Conflict of interest: Maria Gheorge, Roberto Di Virgilio, Maria Alejandra Vidal Pereira and Michal Kantecki are employees of Pfizer and hold stock options. Fionn Woodcock and Xiyu Bao are employees of Source Health Economics, a consultancy company that received funding from Pfizer to provide health economic and writing services for this project. Marco Falcone has received research grants from MSD, Gilead, and Menarini and participated in advisory boards or received speaker fees from Pfizer, Menarini, Thermo Fisher, Mundipharma and Shionogi. Ethics approval: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. Consent to participate: Not applicable. Consent for publication: Not applicable. Availability of data and material: All data generated or analysed during this study are included in this published article as supplementary information files. Code availability: Not applicable. Authors’ contributions: All authors: study concept and design, acquisition of data and critical revision of the manuscript. All authors have given approval to the final version of the manuscript. MG: analysis and interpretation of data, drafting of the manuscript, obtained funding, administrative, technical, or material support, and study supervision. XB and FW: analysis and interpretation of data, drafting of the manuscript and statistical analysis.
Figures

References
-
- O'Neill J. Tackling drug-resistant infections globally: final report and recommendations. 2016. https://amr-review.org/sites/default/files/160518_Final%20paper_with%20c.... Accessed May 2024.
-
- Ma J, Song X, Li M, Yu Z, Cheng W, Yu Z, et al. Global spread of carbapenem-resistant Enterobacteriaceae: epidemiological features, resistance mechanisms, detection and therapy. Microbiol Res. 2023;266: 127249. 10.1016/j.micres.2022.127249. - PubMed
-
- Lee YL, Chen HM, Hii IM, Hsueh PR. Carbapenemase-producing Enterobacterales infections: recent advances in diagnosis and treatment. Int J Antimicrob Agents. 2022;59(2): 106528. 10.1016/j.ijantimicag.2022.106528. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

