ALKBH5 demethylates the m6A modification of SOCS3 in microglia/macrophages and alleviates neuroinflammation after brain injury
- PMID: 40986354
- PMCID: PMC12501137
- DOI: 10.1073/pnas.2504697122
ALKBH5 demethylates the m6A modification of SOCS3 in microglia/macrophages and alleviates neuroinflammation after brain injury
Abstract
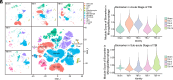
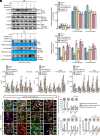
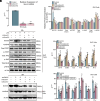
Microglia/macrophage-induced neuroinflammation plays a crucial role in the progression of traumatic brain injury (TBI). However, the involvement of N6-methyladenosine (m6A) RNA modifications in this process remains elusive. Single-cell RNA sequencing (scRNA-seq) and m6A RNA immunoprecipitation sequencing (MeRIP-seq) across multiple time points postinjury revealed a strong correlation between m6A modifications and genes enriched in microglia/macrophages. Furthermore, the m6A demethylase ALKBH5 was identified as a key regulator of dynamic m6A patterns at the injury site. ALKBH5 suppression in microglia/macrophages exacerbated neuroinflammation in vitro and worsened neurological deficits in controlled cortical impact (CCI) models. MeRIP-qPCR and RNA pull-down assays revealed SOCS3 was a downstream target of ALKBH5-mediated m6A demethylation. This demethylation stabilized Socs3 mRNA and enhanced its protein expression, which in turn suppressed neuroinflammation via inhibiting the JAK2-STAT3 pathway. Conversely, SOCS3 depletion impaired functional recovery after injury. These findings unveiled a critical ALKBH5-m6A-SOCS3 regulatory axis that mitigated microglia/macrophage-driven neuroinflammation after TBI, underscoring its potential as a therapeutic intervention target for TBI progression.
Keywords: ALKBH5; N6-methyladenosine; SOCS3; microglia; neuroinflammation.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures




References
-
- Smith D. H., Meaney D. F., Shull W. H., Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 18, 307–316 (2003). - PubMed
-
- Hanisch U.-K., Kettenmann H., Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nature neuroscience 10, 1387–1394 (2007). - PubMed
-
- Panday A., et al. , Transcription factor NF-κB: An update on intervention strategies. Arch. Immunol. Ther. Exp. 64, 463–483 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

