Targeting endothelial ERG to mitigate vascular regression in retinopathies
- PMID: 40986356
- PMCID: PMC12501155
- DOI: 10.1073/pnas.2507194122
Targeting endothelial ERG to mitigate vascular regression in retinopathies
Abstract
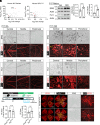
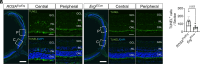
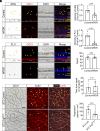
Retinopathy of prematurity (ROP) and diabetic retinopathy (DR) are ocular disorders in which an initial loss of retinal capillaries leads to damaging tissue ischemia followed by a compensatory neovascularization response that generates pathological capillaries in the eye. Using a mouse model of ROP and samples from DR patients, we found that the highly homologous and homeostatic erythroblast transformation-specific (ETS) family transcription factors ETS-related gene (ERG) and Friend leukemia integration 1 (FLI1) are downregulated in endothelial cells (ECs) of retinal capillaries prior to their regression in early stages of these diseases. We developed a mouse model of inducible EC-specific overexpression of Erg and found it mitigates capillary regression, retinal neuron death, neovascularization, and visual defects in the ROP model. Erg overexpression also reduces capillary regression in early stages of a murine DR model. We next found that simultaneous deletion of endothelial Erg and Fli1 is sufficient to promote regression of the pathological retinal capillaries that arise in late stages of the ROP model. Altogether, our data demonstrate that deletion of homeostatic endothelial ETS factors promotes capillary regression, while maintenance of even one of these factors prevents regression. These findings offer insights into approaches for preventing and treating retinopathies at different stages of these diseases.
Keywords: diabetic retinopathy; microvascular rarefaction; retina; retinopathy of prematurity; transcriptional regulator ERG.
Conflict of interest statement
Competing interests statement:E.M., C.M.S., and C.T.G. filed a provisional patent (#63/666,397) related to this work.
Figures




Update of
-
Targeting endothelial ERG to mitigate vascular regression and neuronal ischemia in retinopathies.bioRxiv [Preprint]. 2024 Dec 27:2024.12.27.630529. doi: 10.1101/2024.12.27.630529. bioRxiv. 2024. Update in: Proc Natl Acad Sci U S A. 2025 Sep 30;122(39):e2507194122. doi: 10.1073/pnas.2507194122. PMID: 39763974 Free PMC article. Updated. Preprint.
References
-
- Jampol L. M., Glassman A. R., Sun J., Evaluation and care of patients with diabetic retinopathy. N. Engl. J. Med. 382, 1629–1637 (2020). - PubMed
-
- Hartnett M. E., Pathophysiology of retinopathy of prematurity. Annu. Rev. Vis. Sci. 9, 39–70 (2023). - PubMed
-
- Wong T. Y., Cheung C. M., Larsen M., Sharma S., Simo R., Diabetic retinopathy. Nat. Rev. Dis. Primers 2, 16012 (2016). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

