EML4-ALK variant-specific genetic interactions shape lung tumorigenesis
- PMID: 40986428
- PMCID: PMC12479099
- DOI: 10.1158/2159-8290.CD-24-1417
EML4-ALK variant-specific genetic interactions shape lung tumorigenesis
Abstract
Diverse fusions of EML4 and ALK are oncogenic drivers in lung adenocarcinomas. EML4-ALK variants have distinct breakpoints within EML4, but their functional differences remain poorly understood. Here, we use somatic genome editing to generate autochthonous mouse models of EML4-ALK-driven lung tumors and show that V3 is more oncogenic than V1. By employing multiplexed genome editing and quantifying the effects of 29 putative tumor suppressor genes on V1- and V3-driven lung cancer growth, we show that many tumor suppressor genes have variant-specific effects on tumorigenesis. Pharmacogenomic analyses further suggest that tumor genotype can influence therapeutic responses. Analysis of human EML4-ALK-positive lung cancers also identified variant-specific differences in their genomic landscapes. These findings suggest that EML4-ALK variants behave more like distinct oncogenes rather than a uniform entity and highlight the dramatic impact of oncogenic fusion partner proteins and coincident tumor suppressor gene alterations on the biology of oncogenic fusion-driven cancers.
Conflict of interest statement
Competing interest statement
M.M.W. and D.A.P. are co-founders of, and hold equity in, Guide Oncology, Inc. S.S, S.D.S, and E.S.S., are employees at Foundation Medicine, Inc., with an equity interest in Roche.
Figures



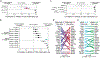


References
-
- Badia-i-Mompel P, Santiago JV, Braunger J, Geiss C, Dimitrov D, Müller-Dott S, Taus P, Dugourd A, Holland CH, Flores ROR, Saez-Rodriguez J (2022) decoupleR: ensemble of computational methods to infer biological activities from omics data. Bioinform Adv 2:vbac016. 10.1093/bioadv/vbac016 - DOI - PMC - PubMed
-
- Blair LM, Juan JM, Sebastian L, Tran VB, Nie W, Wall GD, Gerceker M, Lai IK, Apilado EA, Grenot G, Amar D, Foggetti G, Carmo MD, Ugur Z, Deng D, Chenchik A, Zafra MP, Dow LE, Politi K, MacQuitty JJ, Petrov DA, Winslow MM, Rosen MJ, Winters IP (2023) Oncogenic context shapes the fitness landscape of tumor suppression. Nat Commun 14:6422. 10.1038/s41467-023-42156-y - DOI - PMC - PubMed
-
- Cai H, Chew SK, Li C, Tsai MK, Andrejka L, Murray CW, Hughes NW, Shuldiner EG, Ashkin EL, Tang R, Hung KL, Chen LC, Lee SYC, Yousefi M, Lin W-Y, Kunder CA, Cong L, McFarland CD, Petrov DA, Swanton C, Winslow MM (2021a) A Functional Taxonomy of Tumor Suppression in Oncogenic KRAS–Driven Lung Cancer. Cancer Discov 11:1754–1773. 10.1158/2159-8290.cd-20-1325 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

