Do Allergic Comorbidities Alter the Efficacy and Safety of Abrocitinib or Dupilumab in Patients with Moderate-to-Severe Atopic Dermatitis?
- PMID: 40987931
- PMCID: PMC12550080
- DOI: 10.1007/s13555-025-01516-w
Do Allergic Comorbidities Alter the Efficacy and Safety of Abrocitinib or Dupilumab in Patients with Moderate-to-Severe Atopic Dermatitis?
Abstract
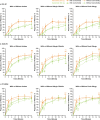
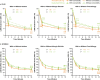
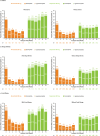
Introduction: Allergic comorbidities are common in patients with atopic dermatitis (AD). Individual trials with abrocitinib or dupilumab demonstrated efficacy and safety in patients with moderate-to-severe AD and allergic comorbidities. This post hoc analysis of the phase 3 JADE COMPARE and DARE trials compared efficacy, safety, and quality of life following abrocitinib and dupilumab treatment in adults with moderate-to-severe AD, with or without comorbid asthma, allergic rhinitis, or food allergy.
Methods: Data were pooled from patients who received abrocitinib (200 mg/day) or dupilumab (300 mg/every 2 weeks) for 16 weeks with concomitant topical therapy. Assessments by self-reported asthma, allergic rhinitis, or food allergy included the proportion of patients achieving Investigator's Global Assessment of clear or almost clear (IGA 0/1), ≥ 75% improvement in Eczema Area and Severity Index (EASI-75), ≥ 4-point improvement in Peak Pruritus Numerical Rating Scale (PP-NRS4), least squares mean change from baseline in Dermatology Life Quality Index (DLQI) and SCORing Atopic Dermatitis (SCORAD), and safety.
Results: Of 1195 patients (abrocitinib, n = 588; dupilumab, n = 607), 377 (32%), 225 (19%), and 211 (18%) patients self-reported comorbid asthma, food allergy, or allergic rhinitis, respectively. Week 16 IGA 0/1 responses were comparable between patients with/without comorbidity with abrocitinib (52%/54% [with/without asthma], 50%/54% [with/without allergic rhinitis], and 53%/53% [with/without food allergy]) or dupilumab (42%/42%, 37%/43%, and 47%/41%). EASI-75 and PP-NRS4 responses and DLQI and SCORAD improvements were also comparable between patients with/without comorbidity in each treatment arm. Treatment-emergent adverse events were more common in patients with comorbidities in the abrocitinib (76%/67% [with/without asthma], 80%/67% [with/without allergic rhinitis], and 78%/67% [with/without food allergy]) and dupilumab (71%/53%, 71%/57%, and 62%/59%) arms.
Conclusion: Abrocitinib and dupilumab improved AD signs and symptoms with a manageable safety profile in patients with moderate-to-severe AD, regardless of asthma, allergic rhinitis, or food allergy. Graphical Abstract available for this article.
Trial registration: ClinicalTrials.gov identifier, NCT03720470 (JADE COMPARE) and NCT04345367 (DARE).
Keywords: Abrocitinib; Allergic rhinitis; Asthma; Comorbidity; Dupilumab; Food allergy; Moderate-to-severe atopic dermatitis; Pruritus; Quality of life.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of Interest: Eric L. Simpson received grants from Pfizer, Eli Lilly, Kyowa Kirin, LEO Pharma, Merck, and Regeneron and personal fees from Pfizer, Bausch Health (Valeant), Dermira, Eli Lilly, Galderma, LEO Pharma, Menlo Therapeutics, Novartis, Regeneron, and Sanofi Genzyme. Jonathan I. Silverberg served as an investigator for Celgene, Eli Lilly, F. Hoffmann-La Roche, Menlo Therapeutics, Realm Therapeutics, Regeneron, and Sanofi Genzyme; as a consultant for Pfizer, AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Dermavant, Dermira, Eli Lilly, Galderma, GlaxoSmithKline, Glenmark, Incyte, Kiniksa Pharmaceuticals, LEO Pharma, Menlo Therapeutics, Novartis, Realm Therapeutics, Regeneron, and Sanofi Genzyme; and as a speaker for Regeneron and Sanofi Genzyme. Bob Geng has worked as a consultant for Pfizer and Genentech; as a speaker/consultant for Regeneron, Sanofi Genzyme, CSL Behring, and Horizon Therapeutics; and is on advisory boards for Novartis and Shire. José-Manuel Carrascosa has participated as an invited speaker, primary or secondary investigator in clinical trials, and advisor for Sanofi, LEO Pharma, Novartis, Almirall, Eli Lilly, Janssen, AbbVie, Celgene, Amgen, Mylan, Biogen, Pfizer, and Sandoz, Boehringer Ingelheim, and Bristol Myers Squibb. Thomas Bieber is/was a lecturer and/or consultant for Pfizer, AbbVie, Affibody, Almirall, Amagma Therapeutics, AnaptysBio, AOBiome, Anergis, Apogee, Arena, Aristea, Artax, Asana Biosciences, ASLAN Pharma, Astria, Attovia, BambusTx, Bayer Health, BioVersys, Boehringer Ingelheim, Bristol Myers Squibb, Byome Labs, Connect Pharma, Daiichi Sankyo, Dermavant, DICE Therapeutics, Domain Therapeutics, EQRx, Galderma, Galapagos, Gilead, Glenmark, GSK, Incyte, Innovaderm, Janssen, Kirin, Kymab, LEO, LG Chem, Eli Lilly, MSD, Medac, Micreos, Nektar Therapeutics, Novartis, Numab, OM-Pharma, Overtone, Pierre Fabre, Q32bio, RAPT, Samsung Bioepis, Sanofi/Regeneron, TIRmed, UCB, Union Therapeutics, Upstream Bio, and Yuhan. Patrick M. Brunner has received personal fees from Pfizer, AbbVie, Almirall, Amgen, Arena Pharma, Biotest, Boehringer Ingelheim, Celgene, Eli Lilly, GSK, Janssen, LEO Pharma, Novartis, Regeneron, Sanofi Genzyme, and UCB and is an investigator for Pfizer (grant paid to his institution). Delphine Staumont-Sallé has served as a consultant, scientific adviser, and/or clinical study investigator for Pfizer, AbbVie, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Galderma, Janssen, LEO Pharma, Novartis, Sanofi Genzyme, and UCB. Chao Ji has no conflicts to disclose. Pinaki Biswas, Irene Hernández-Martín, and Herwig Koppensteiner are employees and shareholders of Pfizer Inc. Claire Feeney and Francisco José Rebollo Laserna were employees of Pfizer Inc. at the time this study was conducted. Ethical Approval: The JADE DARE and JADE COMPARE trials were conducted in accordance with the principles of the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice guidelines. All local regulatory requirements were followed. The JADE DARE and DARE COMPARE trials were approved by the institutional review board or ethics committee at each of the investigational centers participating in the studies. All patients provided written informed consent.
Figures
References
-
- Boguniewicz M, Fonacier L, Guttman-Yassky E, Ong PY, Silverberg J, Farrar JR. Atopic dermatitis yardstick: practical recommendations for an evolving therapeutic landscape. Ann Allergy Asthma Immunol. 2018;120:10–22 (e2). - PubMed
-
- Silverberg JI. Comorbidities and the impact of atopic dermatitis. Ann Allergy Asthma Immunol. 2019;123:144–51. - PubMed
-
- Ali Z, Egeberg A, Thyssen JP, Ulrik CS, Thomsen SF. Adults with concomitant atopic dermatitis and asthma have more frequent urgent healthcare utilization and less frequent scheduled follow-up visits than adults with atopic dermatitis or asthma only: a nationwide cohort study. J Eur Acad Dermatol Venereol. 2022;36:2406–13. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

