Case report series: Pembrolizumab and enfortumab vedotin in plasmacytoid urothelial carcinoma
- PMID: 40989700
- PMCID: PMC12451449
- DOI: 10.3389/fonc.2025.1608291
Case report series: Pembrolizumab and enfortumab vedotin in plasmacytoid urothelial carcinoma
Abstract
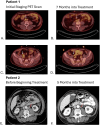
Plasmacytoid urothelial carcinoma (PUC) is a rare and aggressive histologic subtype of urothelial carcinoma with no well-established treatment. Recently, the combination of pembrolizumab and enfortumab vedotin has become the standard of care for locally advanced and metastatic urothelial carcinoma due to improved survival outcomes in the EV-302 trial, but the number of histological subtypes in this trial is unknown. This case series presents three patients with Stage IV PUC who were treated with the combination of pembrolizumab and enfortumab vedotin. Two of the three patients demonstrated sustained stable disease after eight and ten months of treatment with this combination with manageable adverse effects including rash and colitis. The third patient experienced disease progression to leptomeningeal involvement eight months following initial diagnosis and subsequently succumbed to the disease. These observations support the potential efficacy of pembrolizumab in combination with enfortumab vedotin as a therapeutic option for this aggressive urothelial carcinoma subtype.
Keywords: antibody drug conjugate; enfortumab vedotin; immune-checkpoint inhibitors; pembrolizumab; plasmacytoid; urothelial carcinoma.
Copyright © 2025 Bowen, Allison, Hensley and Myint.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

