Effectiveness of oral semaglutide versus empagliflozin for the management of type 2 diabetes. PIONEER-2 trial emulation with real-world data
- PMID: 40990044
- PMCID: PMC12587239
- DOI: 10.1111/dom.70151
Effectiveness of oral semaglutide versus empagliflozin for the management of type 2 diabetes. PIONEER-2 trial emulation with real-world data
Abstract
Background and aims: Oral semaglutide and empagliflozin are commonly used for the management of type 2 diabetes (T2D), but head-to-head comparisons in real-world settings are limited. We aimed to emulate the PIONEER-2 trial using electronic health records to compare the effectiveness and persistence of oral semaglutide versus empagliflozin.
Methods: This was a retrospective multicentre study using electronic health records from Italian diabetes clinics. New users of oral semaglutide or empagliflozin were matched 1:2 and followed for up to 18 months. The primary outcome was HbA1c change; secondary outcomes included weight change and treatment persistence. Analyses used mixed models for repeated measures under both treatment policy and trial product estimands.
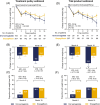
Results: After matching, we included new users of oral semaglutide (n = 105) or empagliflozin (n = 207). Mean age was 65 years, diabetes duration 10 years, baseline HbA1c 7.6%, BMI 29 kg/m2, and 94% were on metformin. Only 28.6% of new users of oral semaglutide reached the 14 mg dose and 31.4% of empagliflozin new users reached the 25 mg dose. HbA1c reduction was significantly greater with oral semaglutide than with empagliflozin (mean difference - 0.35%, p <0.001). Weight loss over time was similar, with oral semaglutide showing a modest advantage at 18 months among persistent patients. Persistence was lower for semaglutide (HR for discontinuation 1.47, p = 0.007).
Conclusions: Under routine care, new users of oral semaglutide achieved better glycaemic control compared with empagliflozin new users, with similar weight loss but lower treatment persistence. These findings support the results of PIONEER-2 and its transferability to clinical practice.
Keywords: cohort study; empagliflozin; real‐world evidence; semaglutide.
© 2025 The Author(s). Diabetes, Obesity and Metabolism published by John Wiley & Sons Ltd.
Conflict of interest statement
GPF received honoraria, lecture, or advisory board fees from AstraZeneca, Boehringer, Guidotti, Lilly, Novartis, Novo Nordisk, and Sanofi. AG has received lecture fees from AstraZeneca, Lilly, and Novo Nordisk. GP reports consulting, advisory board, or speaking honoraria from Eli Lilly, consulting or participation on advisory boards for Bayer, and consulting for Novo Nordisk. AA received research grants, lecture, or advisory board fees from Merck Sharp & Dohme, AstraZeneca, Novartis, Boehringer‐Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Lilly, Servier, and Takeda. AS served on the advisory board of Novo Nordisk, Sankyo, and Sanofi and received speaker fees from AstraZeneca, Bayer, Lilly, Novo Nordisk, and Sanofi. AC received grants from AstraZeneca, Lilly, and Novo Nordisk. He also received speaker fees and provided advisory board services for Abbott, AstraZeneca, Boehringer Ingelheim Pharmaceuticals, Lilly, Merck Sharp & Dohme, Menarini, Novo Nordisk, Sanofi, Sigma‐Tau, and Takeda. EL, SP, MG, MS, and MT have nothing to disclose.
Figures

References
-
- Dinesh Shah A, Langenberg C, Rapsomaniki E, et al. Type 2 diabetes and incidence of a wide range of cardiovascular diseases: a cohort study in 1.9 million people. Lancet. 2015;385(Suppl 1):S86. - PubMed
-
- Longato E, Di Camillo B, Sparacino G, Saccavini C, Avogaro A, Fadini GP. Diabetes diagnosis from administrative claims and estimation of the true prevalence of diabetes among 4.2 million individuals of the Veneto region (north East Italy). Nutr Metab Cardiovasc Dis. 2020;30(1):84‐91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

