Phylogenomics Untangles the Metabolic Potential of Picochlorum tauri, a New Picoalgal Species Causing a Winter Bloom in the Mediterranean Thau Lagoon
- PMID: 40990230
- PMCID: PMC12458973
- DOI: 10.1111/1462-2920.70174
Phylogenomics Untangles the Metabolic Potential of Picochlorum tauri, a New Picoalgal Species Causing a Winter Bloom in the Mediterranean Thau Lagoon
Abstract
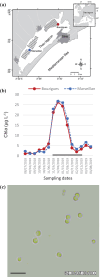
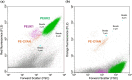
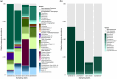
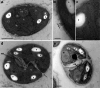
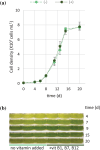
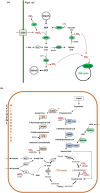
In the winter of 2018-2019, the Mediterranean Thau lagoon experienced an intense green bloom with severe ecological consequences. Here, we aimed at identifying the blooming species and deciphering its metabolic potential. The blooming alga was identified by a metabarcoding approach and later isolated in an axenic form. High-quality nuclear and organellar genome sequences were generated. Phylogenetic and phylogenomic analyses revealed that the alga is a new member of the genus Picochlorum (Trebouxiophyceae, Chlorophyta) that we named Picochlorum tauri. Comparative genomic analyses were conducted to provide insights into (i) genome reduction in the Picochlorum genus with respect to other trebouxiophycean genera and (ii) the metabolic specificities of P. tauri with respect to other eukaryotic picophytoplankton. Genome mining unveiled in P. tauri an extended gene repertoire for carbon concentrating mechanisms, a reduced number of routes for acetyl-CoA synthesis from pyruvate and citrate, and a vitamin B12-dependent carboxylation pathway for propionyl-CoA breakdown. By contrast to the surveyed photosynthetic picoeukaryotes, P. tauri has specific functional traits linked to carbon metabolism, vitamin and chlorophyll synthesis, which are expected to boost physiology. These traits might have contributed to the fast development and maintenance of P. tauri in cool waters under low solar radiance.
Keywords: carbon metabolism; marine algae; metabolic plasticity; photosynthetic picoeukaryote; propionyl‐CoA; trebouxiophyceans; vitamin.
© 2025 The Author(s). Environmental Microbiology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Almagro Armenteros, J. J. , Sønderby C. K., Sønderby S. K., Nielsen H., and Winther O.. 2017. “DeepLoc: Prediction of Protein Subcellular Localization Using Deep Learning.” Bioinformatics 33: 3387–3395. - PubMed
-
- Aminot, A. , and Kérouel R.. 2004. “Caractéristiques Physicochimiques Majeures.” In Hydrobiologie Des écosystèmes Marins: Paramètres et Analyses, 37–41. Edition Ifremer.
-
- Anderson, D. M. 1997. “Turning Back the Harmful Red Tide.” Nature 388: 513–514.
-
- Andrews, S. 2010. “FastQC: A Quality Control Tool for High Throughput Sequence Data.”
-
- Armstrong, G. A. 1998. “Greening in the Dark: Light‐Independent Chlorophyll Biosynthesis From Anoxygenic Photosynthetic Bacteria to Gymnosperms.” Journal of Photochemistry and Photobiology B: Biology 43: 87–100.
MeSH terms
Grants and funding
- Centre National de la Recherche Scientifique (CNRS)
- University of Montpellier
- National Research Institute for Agriculture, Food and the Environment (INRAE)
- the Institut Français de Recherche pour l'Exploitation de la Mer (IFREMER)
- Ministère de l'Environnement, de l'Agriculture et de l'Alimentation (DPMA)
LinkOut - more resources
Full Text Sources

