Human CD4+ T cells recognize Mycobacterium tuberculosis-infected macrophages amid broader responses
- PMID: 40990918
- PMCID: PMC12459340
- DOI: 10.1084/jem.20250460
Human CD4+ T cells recognize Mycobacterium tuberculosis-infected macrophages amid broader responses
Abstract
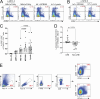
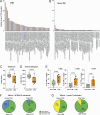
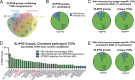
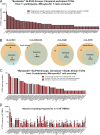
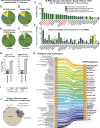
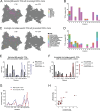
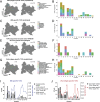
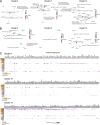
CD4+ T cell-mediated control of tuberculosis (TB) requires recognition of macrophages infected with Mycobacterium tuberculosis (Mtb). Yet, not all Mtb-specific T cells recognize infected macrophages. Using infected monocyte-derived macrophages and autologous memory CD4+ T cells from individuals with stable latent Mtb infection (LTBI), we quantify the frequency of activated T cells. T cell antigen receptor (TCR) sequencing revealed >70% of unique and >90% of total Mtb-specific TCR clonotypes in LTBI are linked to recognition of infected macrophages, while a subset required exogenous antigen exposure, suggesting incomplete recognition. Clonotypes specific for multiple Mtb antigens, and other pathogens, were identified. Remarkably, antigen screening revealed all TCRs to be specific for type VII secretion system (T7SS) substrates. Mtb-specific clonotypes expressed signature effector functions dominated by IFNγ, TNF, IL-2, and GM-CSF or chemokine production and signaling. We propose that TB vaccines, which elicit T cells specific for T7SS substrates, recognize infected macrophages, and express canonical effector functions, will offer protection against TB.
© 2025 Stetsenko et al.
Conflict of interest statement
Disclosures: S.M. Carpenter reported a patent to 63/758,582 pending. No other disclosures were reported.
Figures







Update of
-
Human memory CD4+ T-cells recognize Mycobacterium tuberculosis-infected macrophages amid broader pathogen-specific responses.bioRxiv [Preprint]. 2025 Feb 27:2025.02.23.639515. doi: 10.1101/2025.02.23.639515. bioRxiv. 2025. Update in: J Exp Med. 2025 Dec 1;222(12):e20250460. doi: 10.1084/jem.20250460. PMID: 40060660 Free PMC article. Updated. Preprint.
References
-
- Allan, E.R.O., Tailor P., Balce D.R., Pirzadeh P., McKenna N.T., Renaux B., Warren A.L., Jirik F.R., and Yates R.M.. 2014. NADPH oxidase modifies patterns of MHC class II–restricted epitopic repertoires through redox control of antigen processing. J. Immunol. 192:4989–5001. 10.4049/jimmunol.1302896 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

