Histone Lactylation-Driven Upregulation of VRK1 Expression Promotes Stemness and Proliferation of Glioma Stem Cells
- PMID: 40990975
- PMCID: PMC12667545
- DOI: 10.1002/advs.202503897
Histone Lactylation-Driven Upregulation of VRK1 Expression Promotes Stemness and Proliferation of Glioma Stem Cells
Abstract
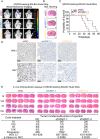
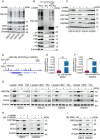
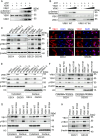
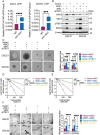
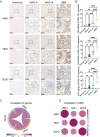
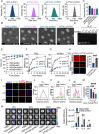
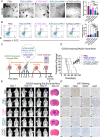
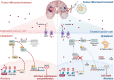
Glioblastoma (GBM) is the most malignant primary brain tumor in adults, with glioma stem cells (GSCs) as a subpopulation contributing to treatment resistance and recurrence. This study investigates the role of lactate and its regulatory gene, vaccinia-related kinase 1 (VRK1), in the regulation of GSC stemness. Utilizing multiple glioma-related databases and patient-derived GSCs, it is discovered that lactate enhances the stemness and proliferation of GSCs via VRK1. Mechanistically, lactate promotes histone lactylation (H3K18la) at the VRK1 promoter in GSCs, thereby upregulating VRK1 expression. VRK1 enhances Y-box binding protein 1 (YBX1) protein stability by inhibiting its ubiquitination and degradation, and phosphorylates YBX1 to promote its nuclear translocation, thereby regulating GSC stemness and proliferation via the YBX1/SOX2 pathway. Additionally, the VRK1-targeted nanoliposome A/TMZ-siVRK1 effectively suppresses the stemness and proliferation of GSCs, demonstrating its therapeutic potential. In conclusion, lactate regulates the stemness and proliferation of GSCs via the H3K18la/VRK1/YBX1/SOX2 pathway. This study elucidates the role of histone lactylation in stem cell regulation and suggests that VRK1 is a potential therapeutic target for GBM.
Keywords: SOX2; VRK1; glioma stem cell; histone lactylation; nanoliposome.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Singh A. K., Arya R. K., Maheshwari S., Singh A., Meena S., Pandey P., Dormond O., Datta D., Int. J. Cancer 2015, 136, 1991. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
