Efficacy and Safety of Lorundrostat in Uncontrolled Hypertension: A Systematic Review and Meta-Analysis
- PMID: 40991241
- PMCID: PMC12459306
- DOI: 10.1111/jch.70155
Efficacy and Safety of Lorundrostat in Uncontrolled Hypertension: A Systematic Review and Meta-Analysis
Abstract
This systematic review and meta-analysis evaluated the efficacy and safety of lorundrostat in adults with uncontrolled hypertension. Following PRISMA guidelines and PROSPERO registration (CRD420251088503), five databases were systematically searched through July 2025 for randomized controlled trials comparing lorundrostat with placebo in this population. The primary outcome was change in systolic blood pressure (SBP), while secondary outcomes included diastolic blood pressure, severe BP events, and adverse effects. Three RCTs comprising 1568 participants across 10 study arms were included. Lorundrostat significantly reduced 24-h ambulatory SBP (mean difference [MD]: -7.45 mmHg; 95% CI: -12.54 to -2.36; p = 0.0041; p2 = 0%) and diastolic BP (MD: -3.49 mmHg; 95% CI: -5.56 to -1.41; p = 0.0010; I2 = 0%). While office SBP showed a non-significant reduction in the primary analysis (MD: -13.55 mmHg; p = 0.077; I2 = 94%), it became statistically significant in a sensitivity analysis (MD: -9.08 mmHg; p < 0.0001). Lorundrostat also significantly lowered the risk of severely elevated BP events (odds ratio [OR]: 0.37; 95% CI: 0.17-0.81; p = 0.028). Adverse effects included an increased risk of hyperkalemia (OR: 3.22; p < 0.001) and hyponatremia (OR: 2.16; p = 0.037), with no significant difference in serious adverse events between groups. In conclusion, lorundrostat demonstrates significant reductions in both ambulatory and diastolic BP in patients with uncontrolled hypertension, with a generally tolerable safety profile. Hyperkalemia and hyponatremia remain notable risks. Further long-term trials are warranted to validate its sustained efficacy and safety.
Keywords: aldosterone synthase inhibitor; blood pressure; lorundrostat; meta‐analysis; randomized controlled trial; systematic review; uncontrolled hypertension.
© 2025 The Author(s). The Journal of Clinical Hypertension published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest related to this study. All authors have reviewed and approved the final manuscript.
Figures






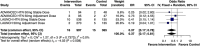





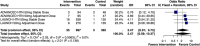


References
-
- Kario K., Okura A., Hoshide S., and Mogi M., “The WHO Global Report 2023 on Hypertension Warning the Emerging Hypertension Burden in Globe and Its Treatment Strategy,” Hypertension Research 47, no. 5 (2024): 1099–1102. - PubMed
-
- Whelton P. K., Carey R. M., Aronow W. S., D. E. Casey Jr , Collins K. J., and Dennison Himmelfarb C., “PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary,” Hypertension 71, no. 6 (2018): e13–e115, 2017ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

