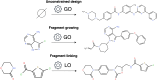
Growing and linking optimizers: synthesis-driven molecule design
- PMID: 40991327
- PMCID: PMC12459256
- DOI: 10.1093/bib/bbaf482
Growing and linking optimizers: synthesis-driven molecule design
Abstract
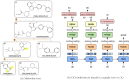
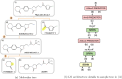
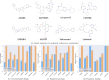
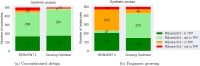
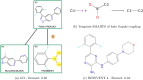
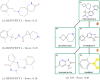
In the present work, two reaction-based generative models for molecular design are presented: growing optimizer and linking optimizer. These models are designed to emulate real-life chemical synthesis by sequentially selecting building blocks and simulating the reactions between them to form new compounds. By focusing on the feasibility of the generated molecules, growing optimizer and linking optimizer offer several advantages, including the ability to restrict chemistry to specific building blocks, reaction types, and synthesis pathways, a crucial requirement in drug design. Unlike text-based models, which construct molecules by iteratively forming a textual representation of the molecular structure, and graph-based models, which assemble molecules atom by atom or fragment by fragment, our approach incorporates a more comprehensive understanding of chemical knowledge, making it relevant for drug discovery projects. Comparative analysis with REINVENT 4, a state-of-the-art molecular generative model, shows that growing optimizer and linking optimizer are more likely to produce synthetically accessible molecules while reaching molecules of interest with the desired properties.
Keywords: deep learning; drug design; generative AI; hit discovery; lead optimization; reinforcement fine tuning.
© The Author(s) 2025. Published by Oxford University Press.
Figures

References
-
- Stanley M, Segler M. Fake it until you make it? Generative de novo design and virtual screening of synthesizable molecules. Curr Opin Struct Biol 2023;82:102658. issn: 0959-440X. 10.1016/j.sbi.2023.102658 . url: https://www.sciencedirect.com/science/article/pii/S0959440X2300132X - DOI - PubMed
-
- Nicolaou CA, Brown N. Multi-objective optimization methods in drug design. Drug Discov Today Technol 2013;10:e427–35. issn: 1740–6749. 10.1016/j.ddtec.2013.02.001 . https://www.sciencedirect.com/science/article/pii/S1740674913000085 - DOI - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

