Mechanistic within-host mathematical model of inhalational anthrax
- PMID: 40991654
- PMCID: PMC12459799
- DOI: 10.1371/journal.pcbi.1013439
Mechanistic within-host mathematical model of inhalational anthrax
Abstract
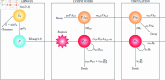
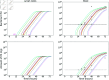
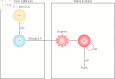
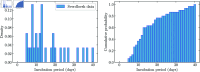
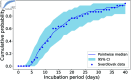
We present a mathematical model of the dynamics of Bacillus anthracis bacteria within the lymph nodes and blood of a host, following inhalation of an initial dose of spores. We also incorporate the dynamics of protective antigen, which is the binding component of the anthrax toxin produced by the bacteria. The model offers a mechanistic description of the early infection dynamics of inhalational anthrax, while its stochastic nature allows us to study the probabilities of different outcomes (for example, how likely it is that the infection will be cleared for a given inhaled dose of spores) in order to explain dose-response data for inhalational anthrax. The model is calibrated via a Bayesian approach, using in vivo data from New Zealand white rabbit and guinea pig infection studies, enabling within-host parameters to be estimated. We also leverage incubation-period data from the Sverdlovsk 1979 anthrax outbreak to show that the model can accurately describe human time-to-symptoms data under reasonable parameter regimes. Finally, we derive a simple approximate formula for the probability of symptom onset before time t, assuming that the number of inhaled spores has a Poisson distribution.
Copyright: © 2025 Whaler et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Cleret A, Quesnel-Hellmann A, Vallon-Eberhard A, Verrier B, Jung S, Vidal D, et al. Lung dendritic cells rapidly mediate anthrax spore entry through the pulmonary route. J Immunol. 2007;178(12):7994–001. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

