Gut epithelium modifies enteric behaviors during nutritional adversity via distinct peptidergic signaling axes
- PMID: 40991693
- PMCID: PMC12459421
- DOI: 10.1126/sciadv.adw1270
Gut epithelium modifies enteric behaviors during nutritional adversity via distinct peptidergic signaling axes
Abstract
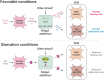
Interorgan signaling events are emerging as key regulators of behavioral plasticity. The foregut and hindgut circuits of the C. elegans enteric nervous system (ENS) control feeding and defecation behavior, respectively. Here, we show that epithelial cells in the midgut integrate feeding state information to control these behavioral outputs by releasing distinct neuropeptidergic signals. In favorable conditions, insulin and noninsulin peptides released from midgut epithelia activate foregut and hindgut enteric neurons, respectively, to sustain normal feeding and defecation behavior. During food scarcity, altered insulin signaling from sensory neurons activates the transcription factor DAF-16/FoxO in midgut epithelia, which blocks both peptidergic signaling axes to the ENS by transcriptionally shutting down the intestinal neuropeptide secretion machinery. Our findings demonstrate that midgut epithelial cells act as integrators relaying internal state information to distinct parts of the ENS to control animal behavior.
Figures






References
-
- M. D. Gershon, The Second Brain (HarperCollins, 1998).
-
- Wood J. D., Enteric nervous system: Reflexes, pattern generators and motility. Curr. Opin. Gastroenterol. 24, 149–158 (2008). - PubMed
-
- Schneider S., Wright C. M., Heuckeroth R. O., Unexpected roles for the second brain: Enteric nervous system as master regulator of bowel function. Annu. Rev. Physiol. 81, 235–259 (2019). - PubMed
-
- Sharkey K. A., Mawe G. M., The enteric nervous system. Physio.l Rev. 103, 1487–1564 (2023).
-
- Drokhlyansky E., Smillie C. S., Van Wittenberghe N., Ericsson M., Griffin G. K., Eraslan G., Dionne D., Cuoco M. S., Goder-Reiser M. N., Sharova T., Kuksenko O., Aguirre A. J., Boland G. M., Graham D., Rozenblatt-Rosen O., Xavier R. J., Regev A., The human and mouse enteric nervous system at single-cell resolution. Cell 182, 1606–1622.e23 (2020). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

